This series of illustrations shows how metabolic acidosis develops at the cellular level.
- As hydrogen ions (H+) start to accumulate in the body, chemical buffers (plasma bicarbonate [HCO3-] and proteins) in the cells and extracellular fluid bind with them. No signs are detectable at this stage.
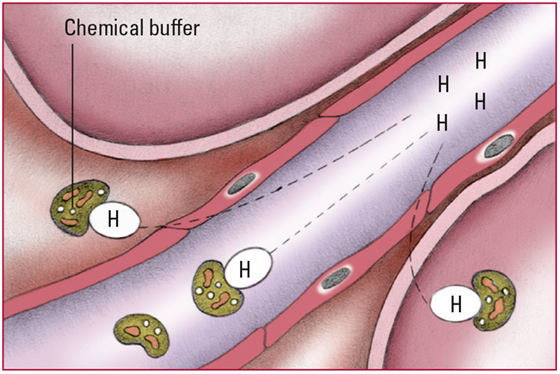
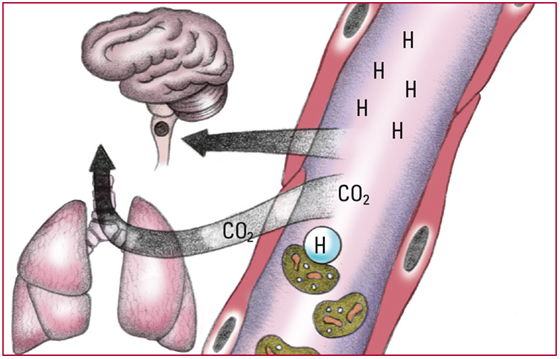
- Excess H+ that can't bind with the buffers decrease pH and stimulate chemoreceptors in the medulla to increase respiratory rate. Increased respiratory rate lowers PaCO2, which allows more H+ to bind with HCO3-. Respiratory compensation occurs within minutes but isn't enough to correct the imbalance. Look for a pH level below 7.35; an HCO3- level below 22 mEq/L; a decreasing PaCO2 level; and rapid, deeper respirations.
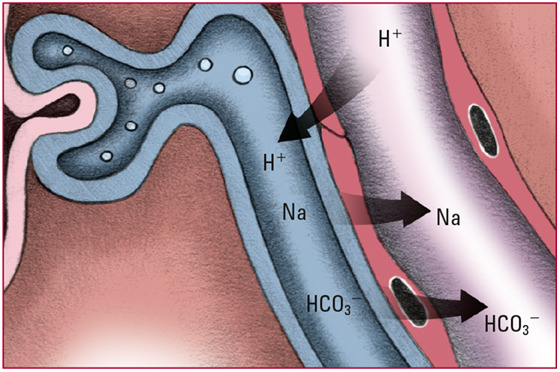
- Healthy kidneys try to compensate for acidosis by secreting excess H+ into the renal tubules. Those ions are buffered by phosphate or ammonia and then are excreted into the urine in the form of a weak acid. Look for acidic urine.
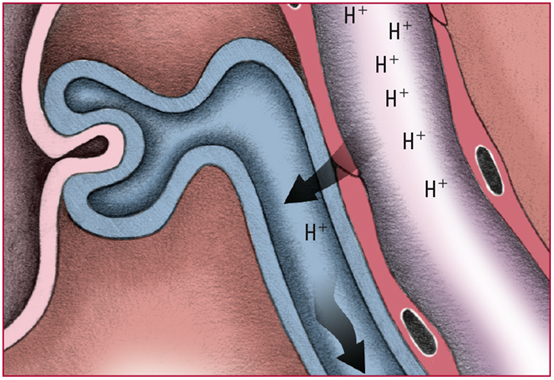
- Each time H+ is secreted into the renal tubules, a sodium ion (Na) and an HCO3- are absorbed from the tubules and returned to the blood. Look for pH and HCO3- levels that slowly return to normal.
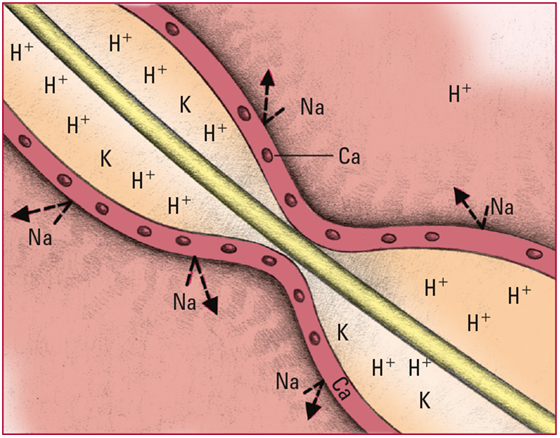
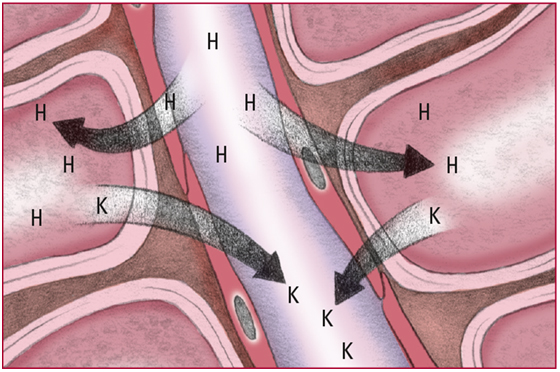
- Excess H+ in the extracellular fluid diffuse into cells. The cells then release potassium ions (K) into the blood to maintain the balance of the charge across the membrane. Look for signs and symptoms of hyperkalemia, including colic and diarrhea, weakness or flaccid paralysis, tingling and numbness in the extremities, bradycardia, a tall T wave, a prolonged PR interval, and a wide QRS complex.
- Excess H+ alter the normal balance of K, Na, and calcium ions (Ca), leading to the reduced excitability of nerve cells. Look for signs and symptoms of progressive CNS depression, including lethargy, dull headache, confusion, stupor, and coma.