This series of illustrations shows how metabolic alkalosis develops at the cellular level.
- As bicarbonate ions (HCO3-) start to accumulate in the body, chemical buffers (in extracellular fluid and cells) bind with them. No signs are detectable at this stage.
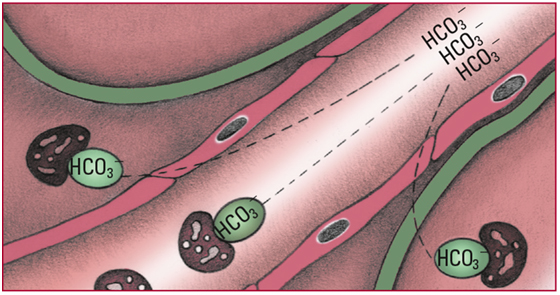
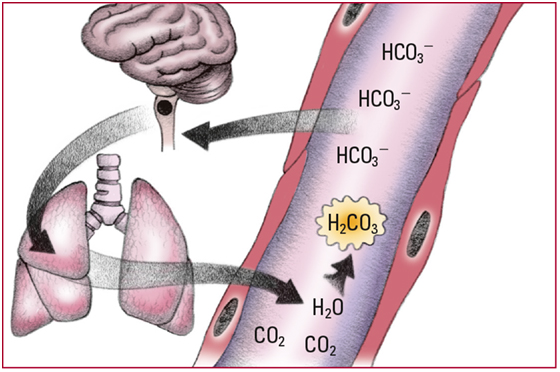
- Excess HCO3- that doesn't bind with chemical buffers elevates serum pH levels, which in turn depresses chemoreceptors in the medulla. Depression of those chemoreceptors causes a decrease in the respiratory rate, which increases PaCO2. The additional carbon dioxide (CO2) combines with water (H2O) to form carbonic acid (H2CO3). Note: Lowered oxygen levels limit respiratory compensation. Look for a serum pH level above 7.45; an HCO3- level above 26 mEq/L; a rising PaCO2; and slow, shallow respirations.
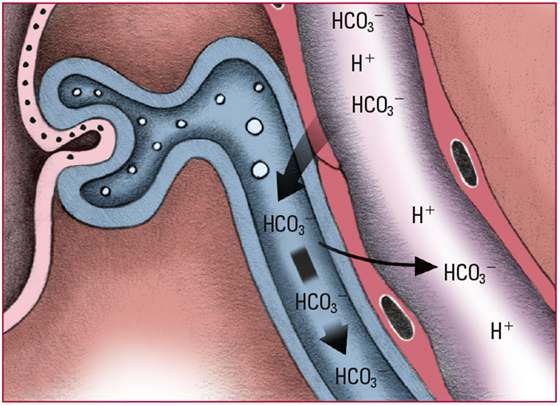
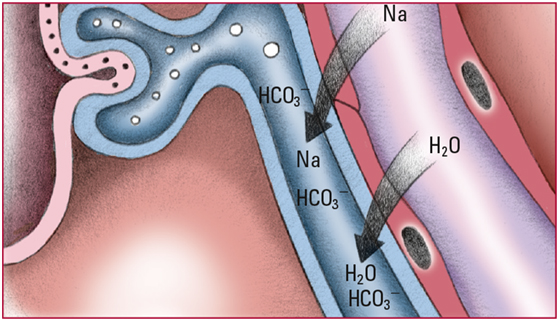
- When the HCO3- level exceeds 28 mEq/L, the renal glomeruli can no longer reabsorb excess amounts. The excess HCO3- is excreted in urine; hydrogen ions (H+) are retained. Look for alkaline urine and pH and HCO3- levels that slowly return to normal.
- To maintain electrochemical balance, the kidneys excrete excess sodium ions (Na), H2O, and HCO3-. Look for polyuria initially and then signs and symptoms of hypovolemia, including thirst and dry mucous membranes.
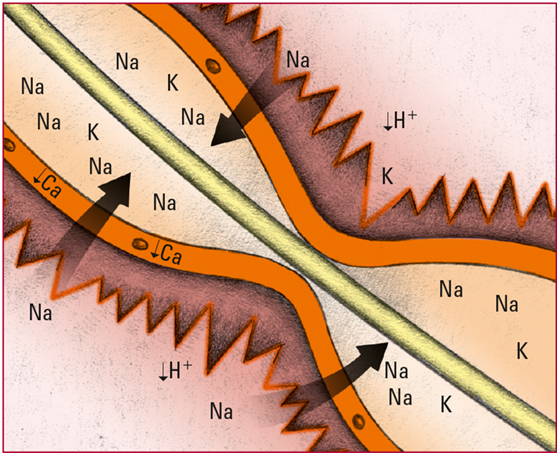
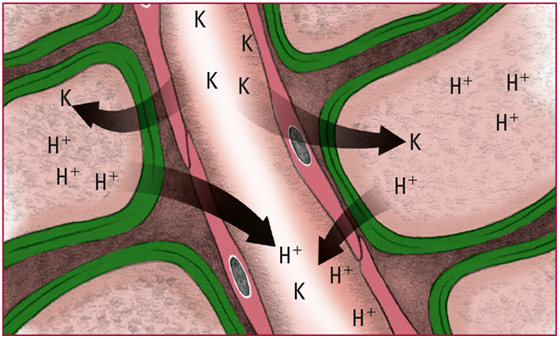
- Lowered H+ levels in the extracellular fluid cause the ions to diffuse out of the cells. To maintain the balance of charge across the cell membrane, extracellular potassium ions (K) move into the cells. Look for signs and symptoms of hypokalemia, including anorexia, muscle weakness, and loss of reflexes.
- As H+ levels decline, calcium (Ca) ionization decreases. That decrease in ionization makes nerve cells more permeable to Na. The movement of Na into nerve cells stimulates neural impulses and produces overexcitability of the peripheral system and CNS. Look for tetany, belligerence, irritability, disorientation, and seizures.