This series of illustrations shows how respiratory acidosis develops at the cellular level.
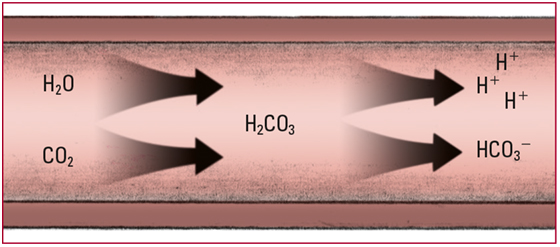
- Pulmonary ventilation decreases, and retained carbon dioxide (CO2) then combines with water (H2O) to form carbonic acid (H2CO3) in larger-than-normal amounts. H2CO3 dissociates to release free hydrogen ions (H+) and bicarbonate ions (HCO3-). Excessive H2CO3 causes a drop in pH. Look for a PaCO2 level above 45 mm Hg and a pH level below 7.35.
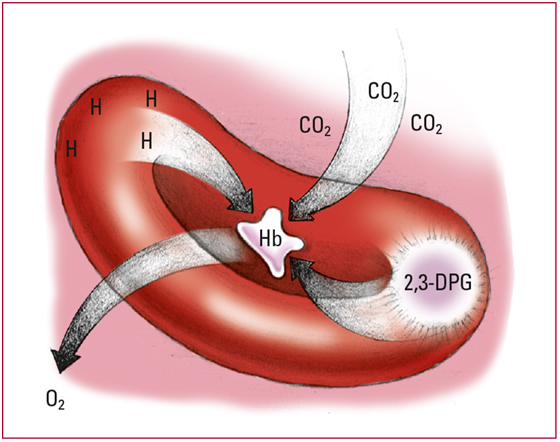
- As the pH level falls, hemoglobin (Hb) release oxygen (O2). The altered Hb, now strongly alkaline, picks up H+ and CO2, thus eliminating some of the free H+ and excess CO2. Look for decreased arterial O2 saturation.
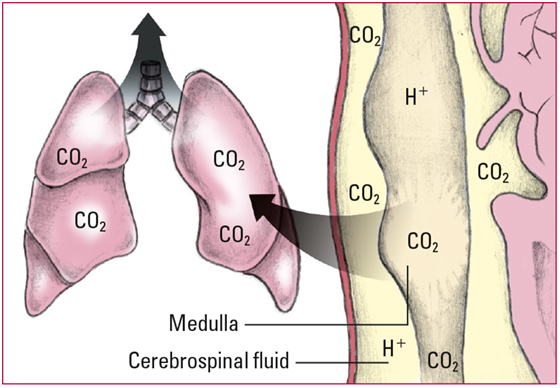
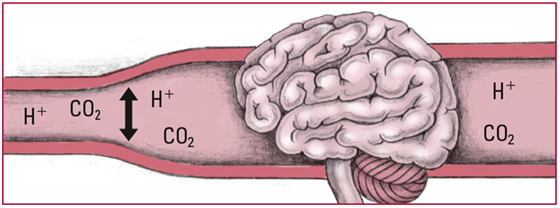
- Whenever PaCO2 increases, CO2 builds up in all tissues and fluids, including cerebrospinal fluid and the respiratory center in the medulla. The CO2 reacts with H2O to form H2CO3, which then breaks into free H+ and HCO3-. The increased amount of CO2 and free H+ stimulates the respiratory center to increase the respiratory rate. An increased respiratory rate expels more CO2 and helps to reduce the CO2 level in blood and tissues. Look for rapid, shallow respirations and a decreasing PaCO2.
- Eventually, CO2 and H+ cause cerebral blood vessels to dilate, which increases blood flow to the brain. That increased flow can cause cerebral edema and depress central nervous system (CNS) activity. Look for a headache, confusion, lethargy, nausea, diaphoresis, tachycardia, and vomiting.
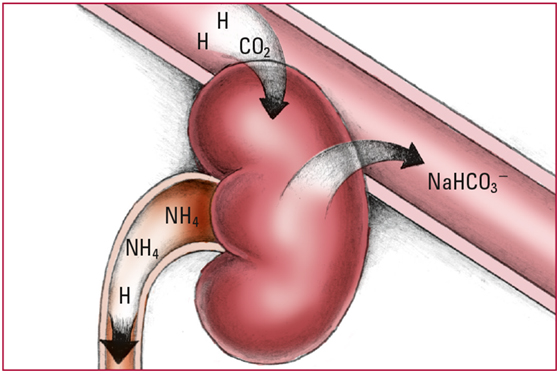
- As respiratory mechanisms fail, the increasing PaCO2 stimulates the kidneys to conserve HCO3- and sodium ions (Na) and to excrete H+, some in the form of ammonium (NH4). The additional HCO3- and Na combines to form extra sodium bicarbonate (NaHCO3-), which is then able to buffer more free H+. Look for increased acid content in the urine; increased serum pH and HCO2- levels; and shallow, depressed respirations.
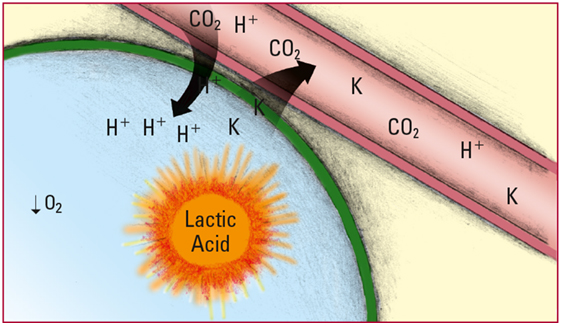
- As the concentration of H+ overwhelms the body's compensatory mechanisms, the H+ move into the cells, and potassium ions (K) move out. A concurrent lack of O2 causes an increase in the anaerobic production of lactic acid, which further skews the acid-base balance and critically depresses neurologic and cardiac functions. Look for hyperkalemia, arrhythmias, increased PaCO2, decreased partial pressure of arterial oxygen (PaO2), decreased pH, and decreased level of consciousness (LOC).