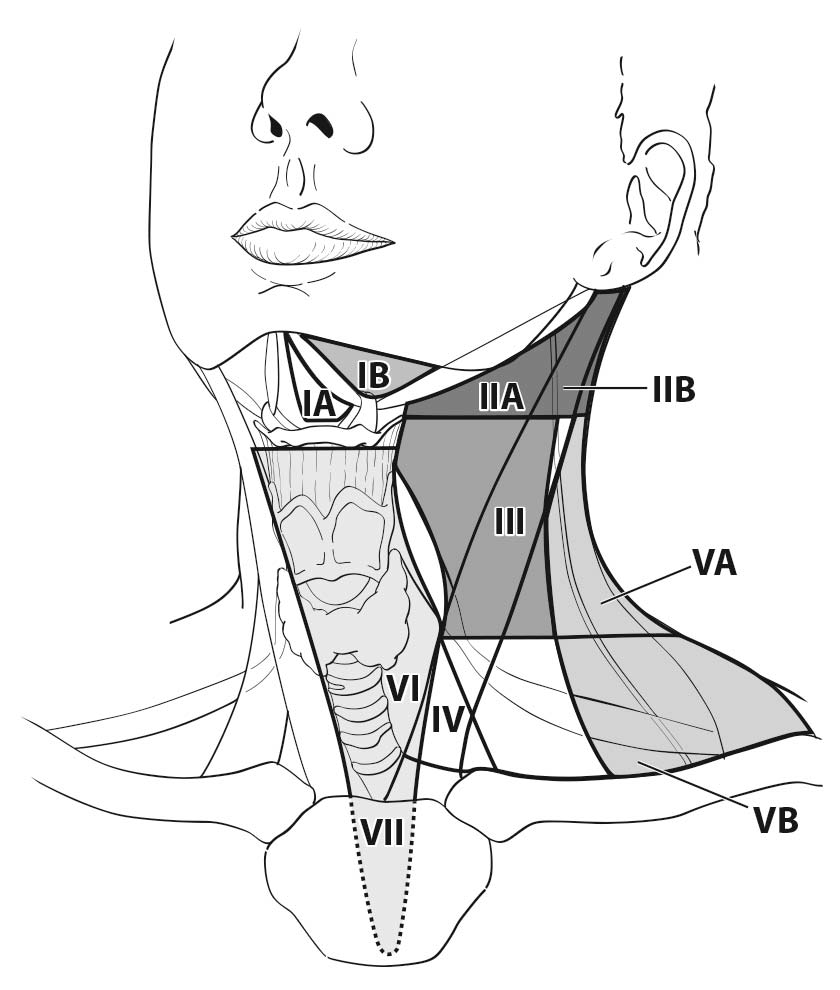
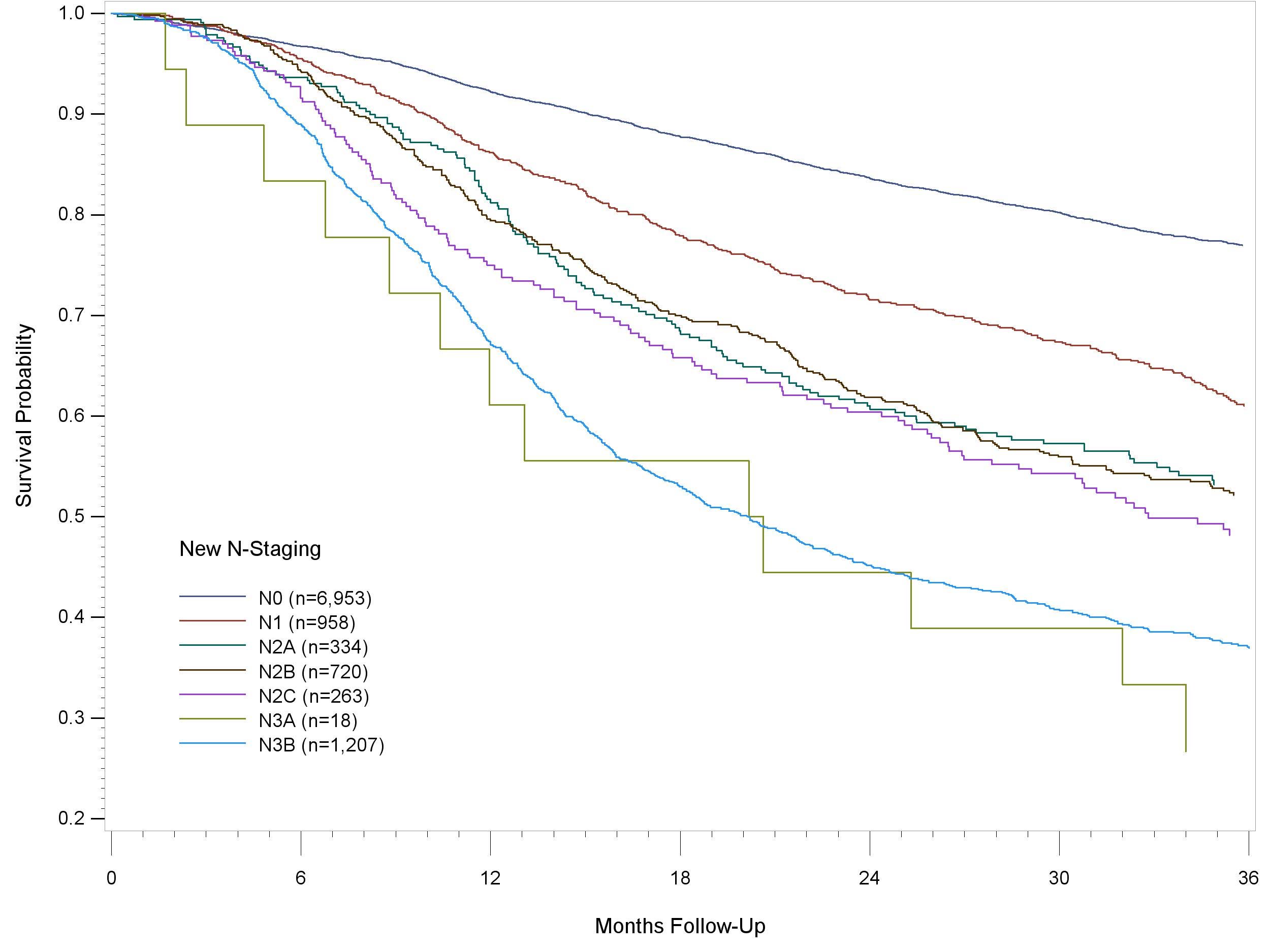
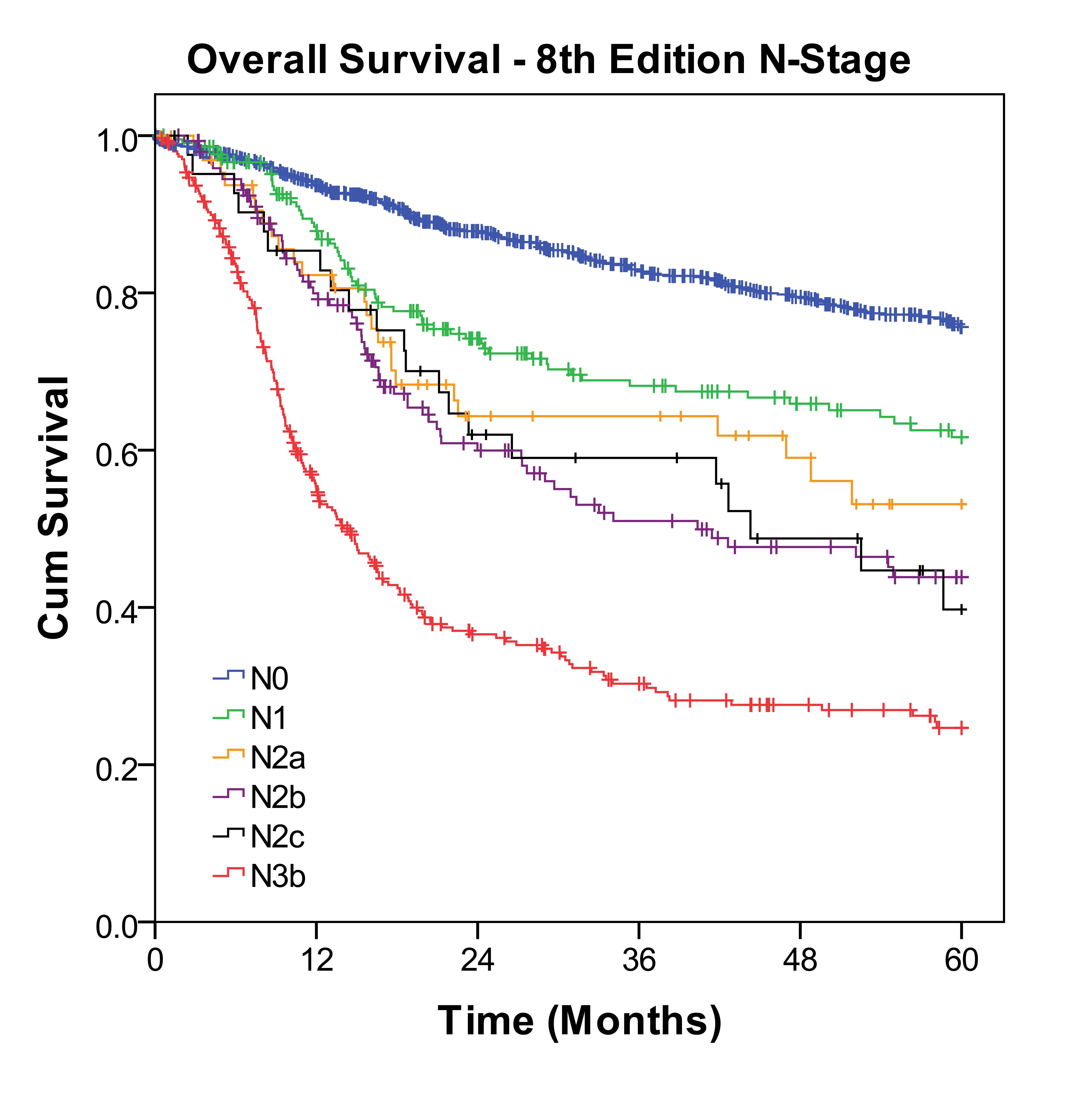
The lymph nodes in the neck may be subdivided into specific anatomic subsites and grouped into seven levels for ease of description (Figure 6.1, Tables 6.1 and 6.2).
Other groups
- Suboccipital
- Retropharyngeal
- Parapharyngeal
- Buccinator (facial)
- Preauricular
- Periparotid and intraparotid
6.1 Anatomical structures defining the boundaries of the neck levels and sublevels.
| Boundary Level | Superior | Inferior | Anterior (medial) | Posterior (lateral) |
|---|---|---|---|---|
| IA | Symphysis of the mand ible | Body of the hyoid | Anterior belly of the contralateral digastric muscle | Anterior belly of the ipsilateral digastric muscle |
| IB | Body of the mand ible | Posterior belly of the diagastric muscle | Anterior belly of the digastric muscle | Stylohyoid muscle |
| IIA | Skull base | Horizontal plane defined by the inferior border of the hyoid bone | Stylohyoid muscle | Vertical plane defined by the spinal accessory nerve |
| IIB | Skull base | Horizontal plane defined by the inferior body of the hyoid bone | Vertical plane defined by the spinal accessory nerve | Lateral border of the sternocleidomastoid muscle |
| III | Horizontal plane defined by the inferior body of the hyoid | Horizontal plane defined by the inferior border of the cricoid cartilage | Lateral border of the sternohyoid muscle | Lateral border of the sternocleidomastoid or sensory branches of the cervical plexus |
| IV | Horizontal plane defined by the inferior border of the cricoid cartilage | Clavicle | Lateral border of the sternohyoid muscle | Lateral border of the sternocleidomastoid or sensory branches of the cervical plexus |
| VA | Apex of the convergence of the sternocleidomastoid and trapezius muscles | Horizontal plane defined by the lower border of the cricoid cartilage | Posterior border of the sternocleido¬mastoid muscle or sensory branches of the cervical plexus | Anterior border of the trapezius muscle |
| VB | Horizontal plane defined by the lower border of the cricoid cartilage | Clavicle | Posterior border of the sternocleido¬mastoid muscle | Anterior border of the trapezius muscle |
| VI | Hyoid bone | Suprasternal notch | Common carotid artery | Common carotid artery |
| VII | Suprasternal notch | Innominate artery | Sternum | Trachea, esophagus, and prevertebral fascia |
| Modified from Robbins KT, Clayman G, Levine PA, et al.,4 with permission of the American Medical Association. | ||||
6.2 Lymph node groups found within the seven levels and sublevels of the neck
| Lymph Node Group | Description |
|---|---|
| Submental (sublevel IA) | Lymph nodes within the triangular boundary of the anterior belly of the digastric muscles and the hyoid bone. These nodes are at greatest risk for harboring metastases from cancers arising from the floor of mouth, anterior oral tongue, anterior mand ibular alveolar ridge, and lower lip. |
| Submand ibular (sublevel IB) | Lymph nodes within the boundaries of the anterior and posterior bellies of the digastric muscle, the stylohyoid muscle, and the body of the mand ible. These include the pregland ular and the postgland ular nodes and the prevascular and postvascular nodes. The sub¬mand ib¬ular gland is included in the specimen when the lymph nodes within the triangle are removed. These nodes are at greatest risk for harboring metastases from cancers arising from the oral cavity, anterior nasal cavity, skin, and soft tissue structures of the midface, as well as from the submand ibular gland . |
| Upper Jugular (sublevels IIA and IIB) | Lymph nodes located around the upper third of the internal jugular vein and adjacent spinal accessory nerve, extending from the level of the skull base (above) to the level of the inferior border of the hyoid bone (below). The anterior (medial) boundary is stylohyoid muscle (the radiologic correlate is the vertical plane defined by the posterior surface of the submand ibular gland ) and the posterior (lateral) boundary is the posterior border of the sternocleido¬mastoid muscle. Sublevel IIA nodes are located anterior (medial) to the vertical plane defined by the spinal accessory nerve. Sublevel IIB nodes are located posterior lateral to the vertical plane defined by the spinal accessory nerve. (The radiologic correlate is the lateral border of the internal jugular on a contrast-enhanced CT scan.) The upper jugular nodes are at greatest risk for harboring metastases from cancers arising from the oral cavity, nasal cavity, nasopharynx, oropharynx, hypopharynx, larynx, and parotid gland . |
| Middle Jugular (level III) | Lymph nodes located around the middle third of the internal jugular vein, extending from the inferior border of the hyoid bone (above) to the inferior border of the cricoid cartilage (below). The anterior (medial) boundary is the lateral border of the sternohyoid muscle, and the posterior (lateral) boundary is the posterior border of the sterno¬cleidomastoid muscle. These nodes are at greatest risk for harboring metastases from cancers arising from the oral cavity, nasopharynx, oropharynx, hypopharynx, and larynx. |
| Lower Jugular (level IV) | Lymph nodes located around the lower third of the internal jugular vein, extending from the inferior border of the cricoid cartilage (above) to the clavicle below. The anterior (medial) boundary is the lateral border of the sternohyoid muscle and the posterior (lateral) boundary is the posterior border of the sternocleido¬mastoid muscle. These nodes are at greatest risk for harboring metastases from cancers arising from the hypopharynx, thyroid, cervical esophagus, and larynx. |
| Posterior Triangle (sublevels VA and VB) | This group is composed predominantly of the lymph nodes located along the lower half of the spinal accessory nerve and the transverse cervical artery. The supraclavicular nodes also are included in the posterior triangle group. The superior boundary is the apex formed by the convergence of the sternocleidomastoid and trapezius muscles; the inferior boundary is the clavicle; the anterior (medial) boundary is the posterior border of the sternocleidomastoid muscle; and the posterior (lateral) boundary is the anterior border of the trapezius muscle. Thus, sublevel VA includes the spinal accessory nodes, whereas sublevel VB includes the nodes following the transverse cervical vessels and the supraclavicular nodes, with the exception of the Virchow node, which is located in level IV. The posterior triangle nodes are at greatest risk for harboring metastases from cancers arising from the nasopharynx, oropharynx, and cutaneous structures of the posterior scalp and neck. |
| Anterior Compartment (level VI) | Lymph nodes in this compartment include the pretracheal and paratracheal nodes, precricoid (Delphian) node, and the perithyroidal nodes, including the lymph nodes along the recurrent laryngeal nerves. The superior boundary is the hyoid bone; the inferior boundary is the suprasternal notch; and the lateral boundaries are the common carotid arteries. These nodes are at greatest risk for harboring metastases from cancers arising from the thyroid gland , glottic and subglottic larynx, apex of the piriform sinus, and cervical esophagus. |
| Superior Mediastinal (level VII) | Lymph nodes in this group include pretracheal, paratracheal, and esophageal groove lymph nodes, extending from the level of the suprasternal notch cephalad and up to the innominate artery caudad. These nodes are at greatest risk of involvement by thyroid cancer and cancer of the esophagus. |
| Modified from Robbins KT, Clayman G, Levine PA, et al.,4 with permission of the American Medical Association. | |