Primary Site(s)
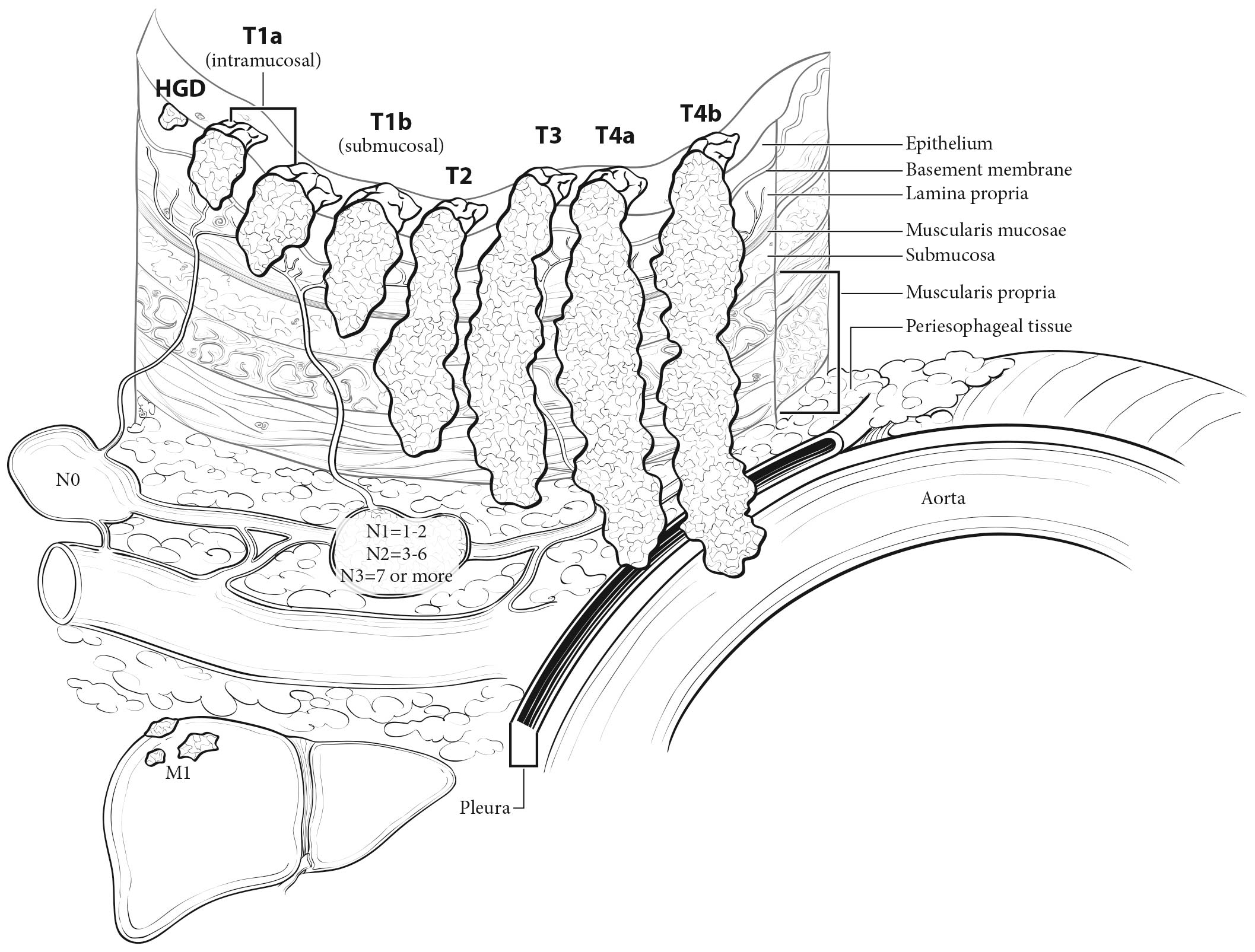
The esophagus traverses three anatomic compartments: cervical, thoracic, and abdominal. The thoracic esophagus is divided arbitrarily into equal thirds: upper, middle, and lower (Table 16.1). However, the clinical importance of the primary site of an esophageal cancer is related less to its position in the esophagus than to its relation to adjacent structures (Figure 16.1).
16.1 Anatomy of esophageal cancer primary site, including typical endoscopic measurements of each region measured from the incisors. Exact measurements depend on body size and height. Location of cancer primary site is defined by cancer epicenter. EGJ, esophagogastric junction; LES, lower esophageal sphincter; UES, upper esophageal sphincter.

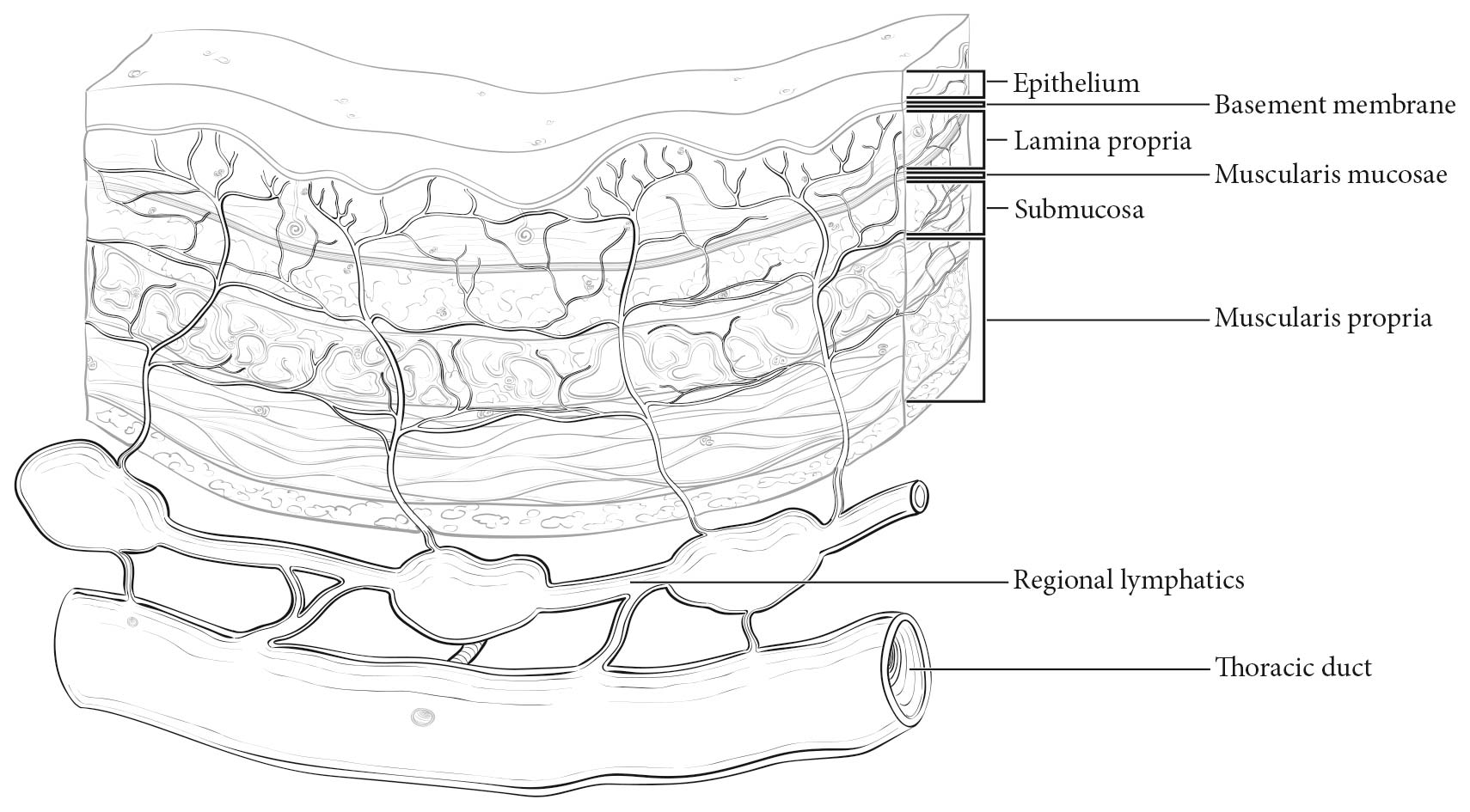
The esophageal wall has three layers: mucosa, submucosa, and muscularis propria (Figure 16.2). The mucosa is composed of epithelium, lamina propria, and muscularis mucosae. A basement membrane isolates the epithelium from the rest of the esophageal wall. In the columnar-lined esophagus, the muscularis mucosae may be a two-layered (duplicated) structure. The clinical importance of this duplicate layer is questionable.7,8 The outer layer is considered the true boundary. The mucosal division may be classified as m1 (epithelium), m2 (lamina propria), or m3 (muscularis mucosae).9 The submucosa has no land marks, but it may be divided into inner (sm1), middle (sm2), and outer (sm3) thirds.9 The muscularis propria has inner circular and outer longitudinal muscle layers. There is no serosa; rather, adventitia (periesophageal connective tissue) lies directly on the muscularis propria.
Location
Cervical Esophagus
Cancers located in the cervical esophagus are staged as upper thoracic esophageal cancers, not as head and neck cancers.
Anatomically, the cervical esophagus lies in the neck, bordered superiorly by the hypopharynx and inferiorly by the thoracic inlet, which lies at the level of the sternal notch. It is subtended by the trachea, carotid sheaths, and vertebrae. Although the length of the esophagus differs somewhat with body habitus, gender, and age, typical endoscopic measurements for the cervical esophagus measured from the incisors are from 15 to less than 20 cm (Figure 16.1). If esophagoscopy is not available, location may be assessed by computed tomography (CT). If the epicenter of the tumor begins above the sternal notch, the location is defined as cervical esophagus.
Upper Thoracic Esophagus
The upper thoracic esophagus is bordered superiorly by the thoracic inlet and inferiorly by the lower border of the azygos vein. Anterolaterally, it is surrounded by the trachea, aortic arch, and great vessels, posteriorly by the vertebrae. Typical endoscopic measurements from the incisor teeth are from 20 to less than 25 cm (Figure 16.1). On CT, to determine the location, the epicenter of an upper thoracic cancer is visible between the sternal notch and the azygos vein.
Middle Thoracic Esophagus
The middle thoracic esophagus is bordered superiorly by the lower border of the azygos vein and inferiorly by the lower border of the inferior pulmonary vein. It is sand wiched between the pulmonary hilum anteriorly, descending thoracic aorta on the left, and vertebrae posteriorly; on the right, it lies freely on the pleura. Typical endoscopic measurements from the incisors are from 25 to less than 30 cm (Figure 16.1). On CT, to determine the location, the epicenter of a middle thoracic cancer is between the azygos vein and the inferior pulmonary vein.
Lower Thoracic Esophagus/Esophagogastic Junction (EGJ)
The lower thoracic esophagus is bordered superiorly by the lower border of the inferior pulmonary vein and inferiorly by the stomach. It is bordered anteriorly by the pericardium, posteriorly by vertebrae, and on the left by the descending thoracic aorta. It normally passes through the diaphragm to reach the stomach, but there is a variable intra-abdominal portion, and in the presence of a hiatal hernia, this portion may be absent. Typical endoscopic measurements from the incisors are from 30 to 40 cm (Figure 16.1). On CT to determine the location, the epicenter of a lower thoracic esophagus/EGJ cancer is below the inferior pulmonary vein. The abdominal esophagus is included in the lower thoracic esophagus. Cancers involving the EGJ that have their epicenter within the proximal 2 cm of the cardia (Siewert types I/II) are to be staged as esophageal cancers. Cancers whose epicenter is more than 2 cm distal from the EGJ, even if the EGJ is involved, will be staged using the stomach cancer TNM and stage groupings (see Chapter 17).
16.1 Anatomy of esophageal cancer primary site by ICD-O-3 topography codes
| Esophageal location | |||||
|---|---|---|---|---|---|
| Anatomic name | Compartment ICD-O-3 | ICD-O-3 | Name | Anatomic boundaries | Typical esophagectomy, cm |
| Cervical | C15.0 | C15.3 | Upper | Hypopharynx to sternal notch | 15 to less than 20 |
| Thoracic | C15.1 | C15.3 | Upper | Sternal notch to azygos vein | 20 to less than 25 |
| C15.4 | Middle | Lower border of azygos vein to inferior pulmonary vein | 25 to less than 30 | ||
| C15.5 | Lower | Lower border of inferior pulmonary vein to EGJ | 30 to less than 40 | ||
| Abdominal | C15.2 | C15.5 | Lower | EGJ to 2 cm below EGJ | 40 to 45 |
| C16.0 | EGJ/cardia | EGJ to 2 cm below EGJ | 40 to 45 | ||
Regional Lymph Nodes
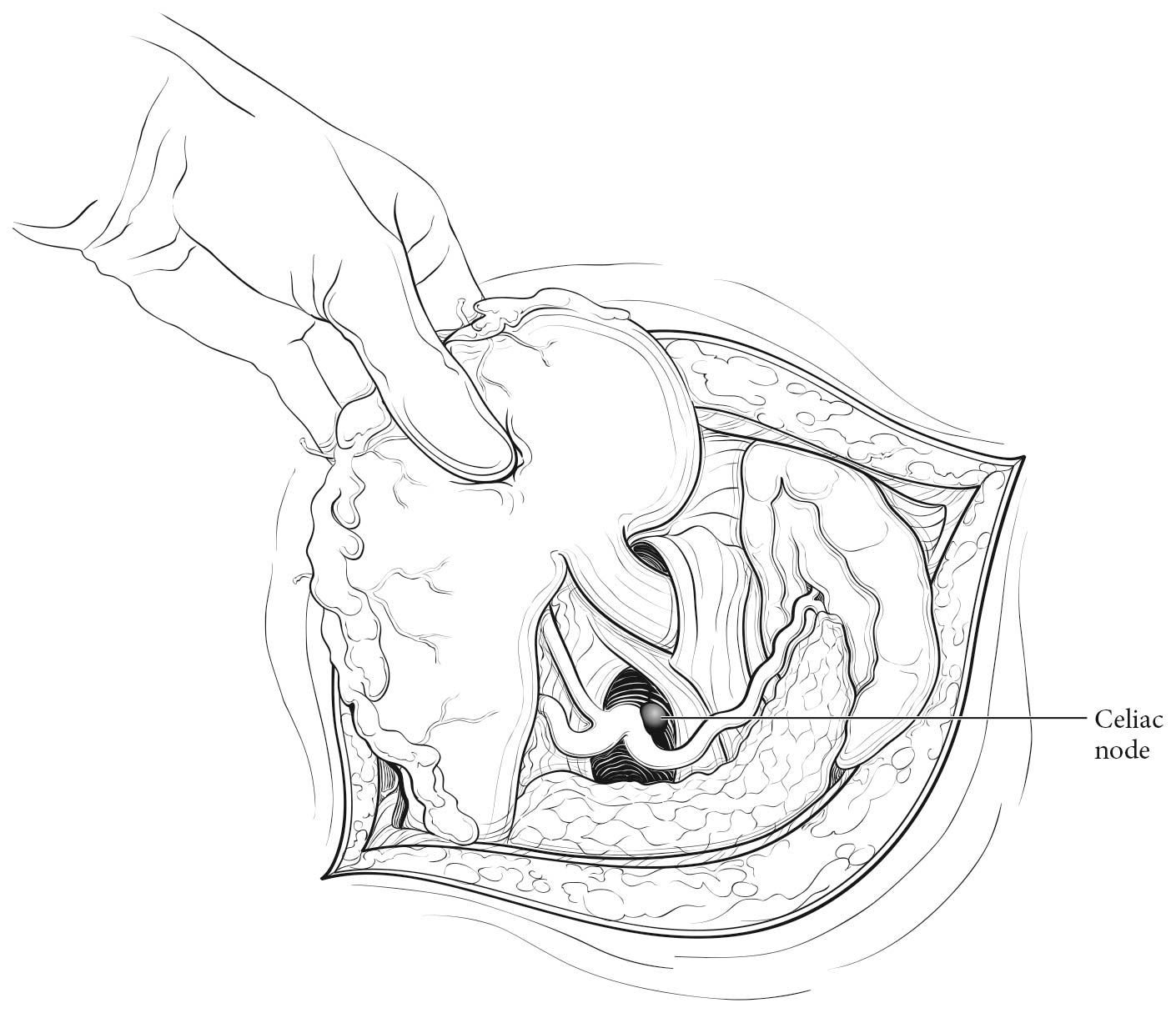
Esophageal lymphatic drainage is intramural and longitudinal. The lymphatic network within the esophagus is concentrated in the submucosa, although lymphatic channels also are present in the lamina propria. This arrangement may permit lymphatic metastases early in the course of the disease from otherwise superficial cancers.10 Lymphatic drainage of the muscularis propria is more limited, but lymphatic channels pierce this layer to drain into regional lymphatic channels and lymph nodes in the periesophageal fat. Up to 43% of autopsy dissections demonstrate direct drainage from the submucosal plexus into the thoracic duct, which facilitates systemic metastases.11-13 The longitudinal nature of the submucosal lymphatic plexus permits lymphatic metastases orthogonal to depth of tumor invasion.14 The implication of the longitudinal nature of lymphatic drainage is that the anatomic site of the cancer and the lymph nodes to which lymphatics drain from that site may not be the same (Figures 16.3_A, 16.3_B, and 16.3_C).
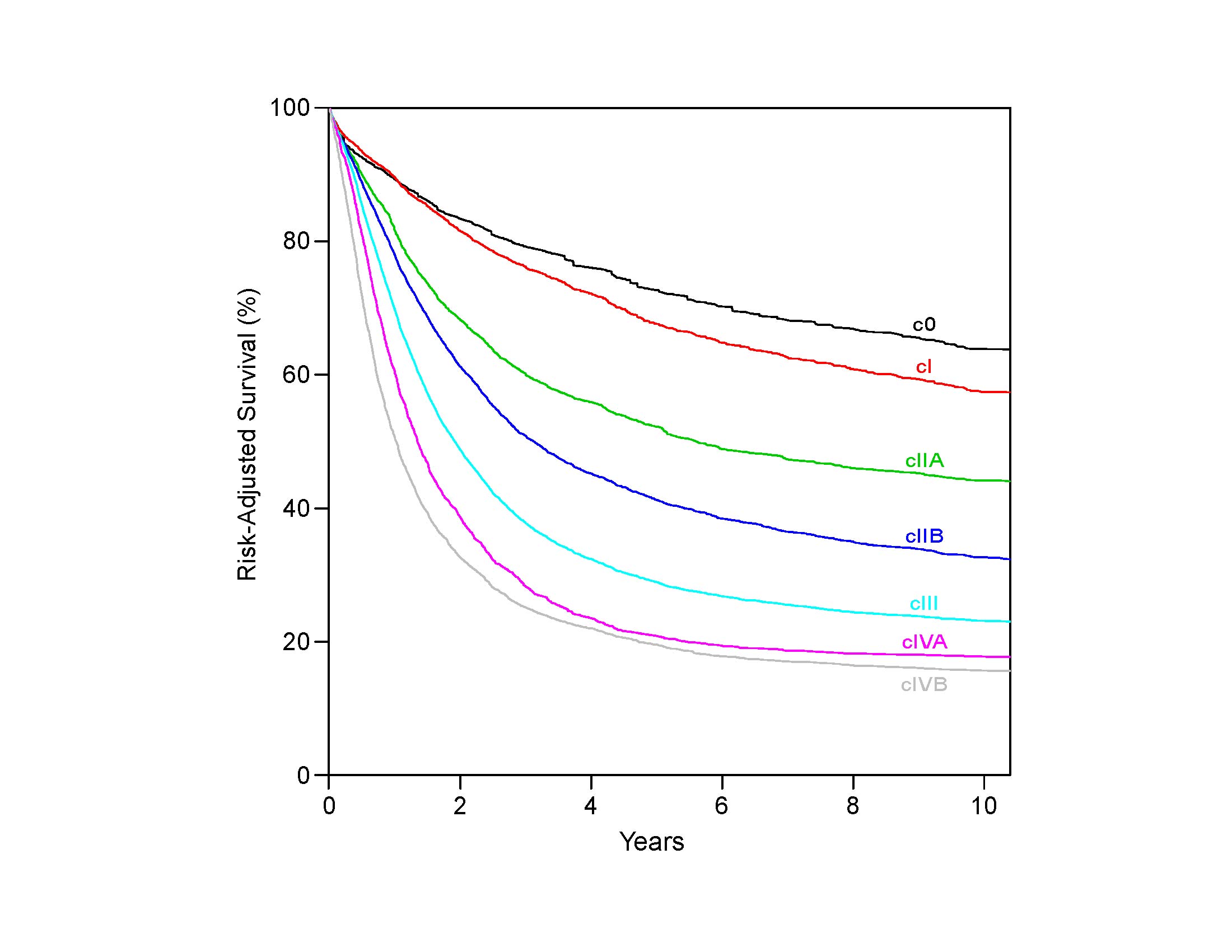
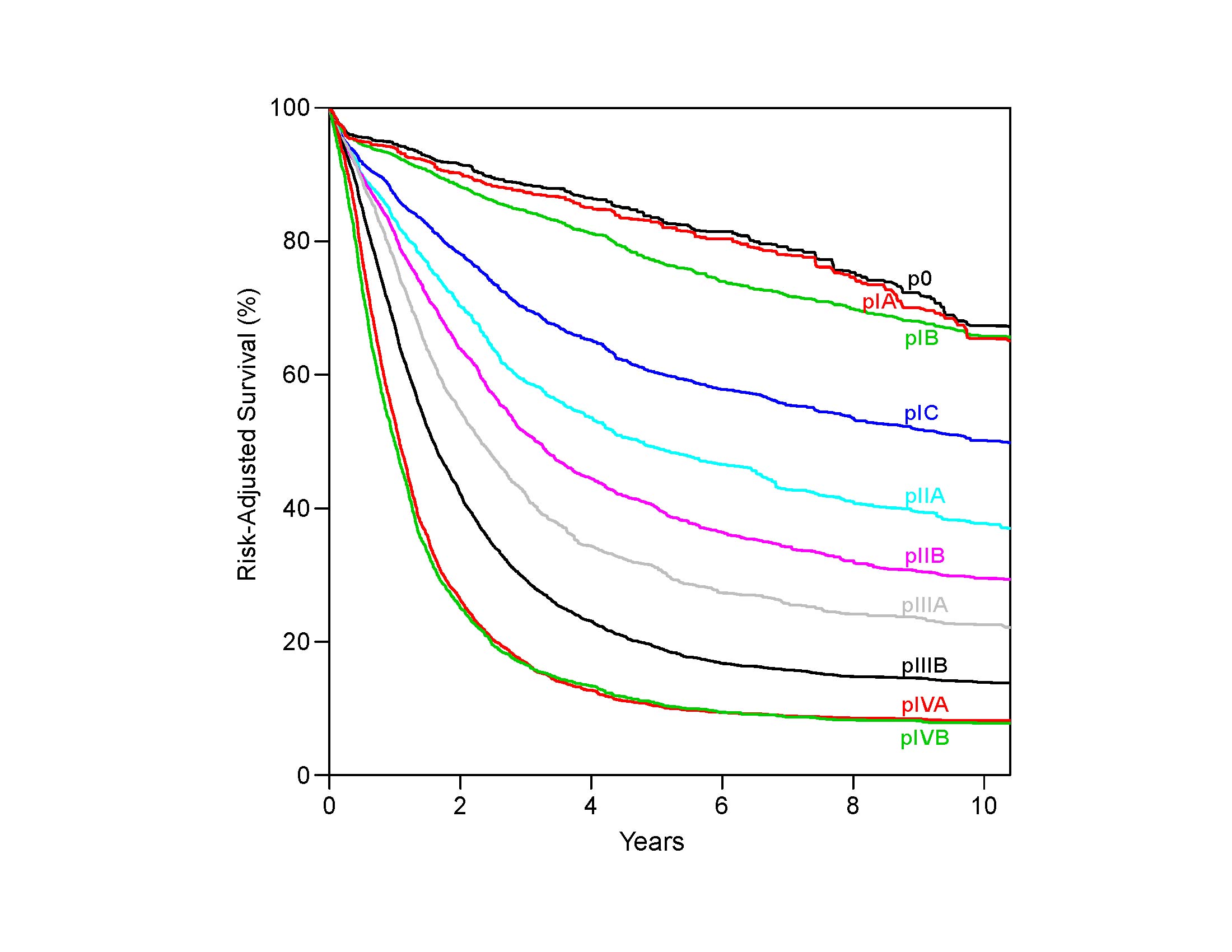
Therefore it follows, and analysis of data supports, that regional lymph nodes for all locations in the esophagus discussed in this chapter extend from periesophageal cervical nodes to celiac nodes (Figures 16.3_A 16.3_B 16.3_Cand 16.4). The nomenclature for thoracic and abdominal regional lymph nodes is listed in Figures 16.3_A, 16.3_B, and 16.3_C. The nomenclature for cervical regional lymph nodes follows that of head and neck chapters (see Chapter 6) and are located in periesophageal levels VI and VII. Lymph nodes in continuity with the esophagus would be considered regional.
The specific regional lymph nodes are as follows:
- Right lower cervical paratracheal nodes: between the supraclavicular paratracheal space and apex of the lung
- Left lower cervical paratracheal nodes: between the supraclavicular paratracheal space and apex of the lung
- Right upper paratracheal nodes: between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung
- Left upper paratracheal nodes: between the top of the aortic arch and apex of the lung
- Right lower paratracheal nodes: between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein
- Left lower paratracheal nodes: between the top of the aortic arch and the carina •Subcarinal nodes: caudal to the carina of the trachea
- Subcarinal nodes: caudal to the carina of the trachea
- Upper thoracic paraesophageal lymph nodes: from the apex of the lung to the tracheal bifurcation
- Middle thoracic paraesophageal lymph nodes: from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein
- Lower thoracic paraesophageal lymph nodes: from the caudal margin of the inferior pulmonary vein to the EGJ
- Pulmonary ligament nodes: within the right inferior pulmonary ligament
- Pulmonary ligament nodes: within the left inferior pulmonary ligament
- Diaphragmatic nodes: lying on the dome of the diaphragm and adjacent to or behind its crura
- Paracardial nodes: immediately adjacent to the gastroesophageal junction
- Left gastric nodes: along the course of the left gastric artery
- Common hepatic nodes: immediately on the proximal common hepatic artery
- Splenic nodes: immediately on the proximal splenic artery
- Celiac nodes: at the base of the celiac artery
- Cervical periesophageal level VI lymph nodes (see Chapter 6)
- Cervical periesophageal level VII lymph nodes (see Chapter 6)
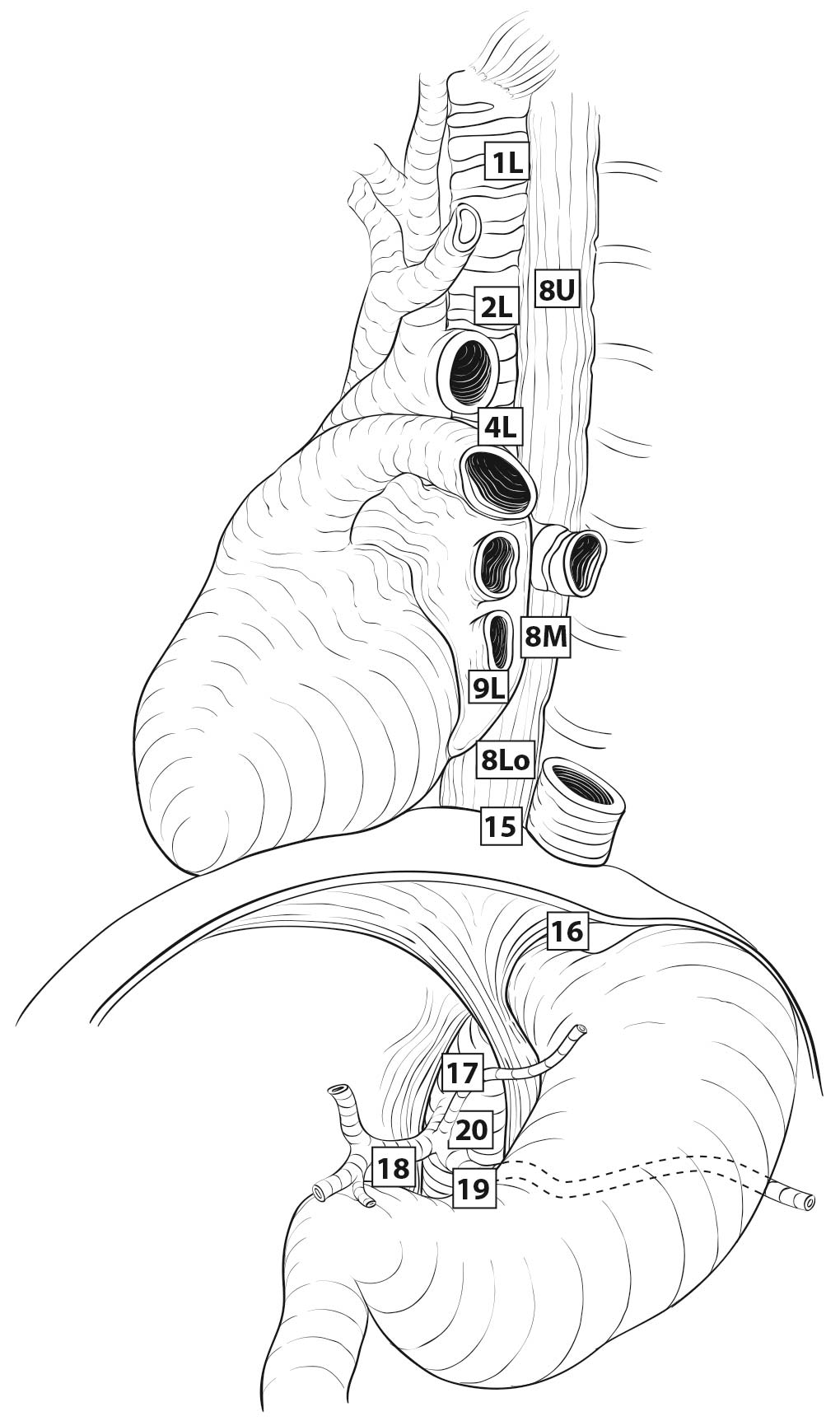
16.3_A (A) Lymph node maps for esophageal cancer. Regional lymph node stations for staging esophageal cancer from left. 1R, Right lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 1L, Left lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 2R, Right upper paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung. 2L, Left upper paratracheal nodes, between the top of the aortic arch and the apex of the lung. 4R, Right lower paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein. 4L, Left lower paratracheal nodes, between the top of the aortic arch and the carina. 7, Subcarinal nodes, caudal to the carina of the trachea. 8U, Upper thoracic paraesophageal lymph nodes, from the apex of the lung to the tracheal bifurcation. 8M, Middle thoracic paraesophageal lymph nodes, from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein. 8Lo, Lower thoracic paraesophageal lymph nodes, from the caudal margin of the inferior pulmonary vein to the EGJ. 9R, Pulmonary ligament nodes, within the right inferior pulmonary ligament. 9L, Pulmonary ligament nodes, within the left inferior pulmonary ligament. 15, Diaphragmatic nodes, lying on the dome of the diaphragm and adjacent to or behind its crura. 16, Paracardial nodes, immediately adjacent to the gastroesophageal junction. 17, Left gastric nodes, along the course of the left gastric artery. 18, Common hepatic nodes, immediately on the proximal common hepatic artery. 19, Splenic nodes, immediately on the proximal splenic artery. 20, Celiac nodes, at the base of the celiac artery.
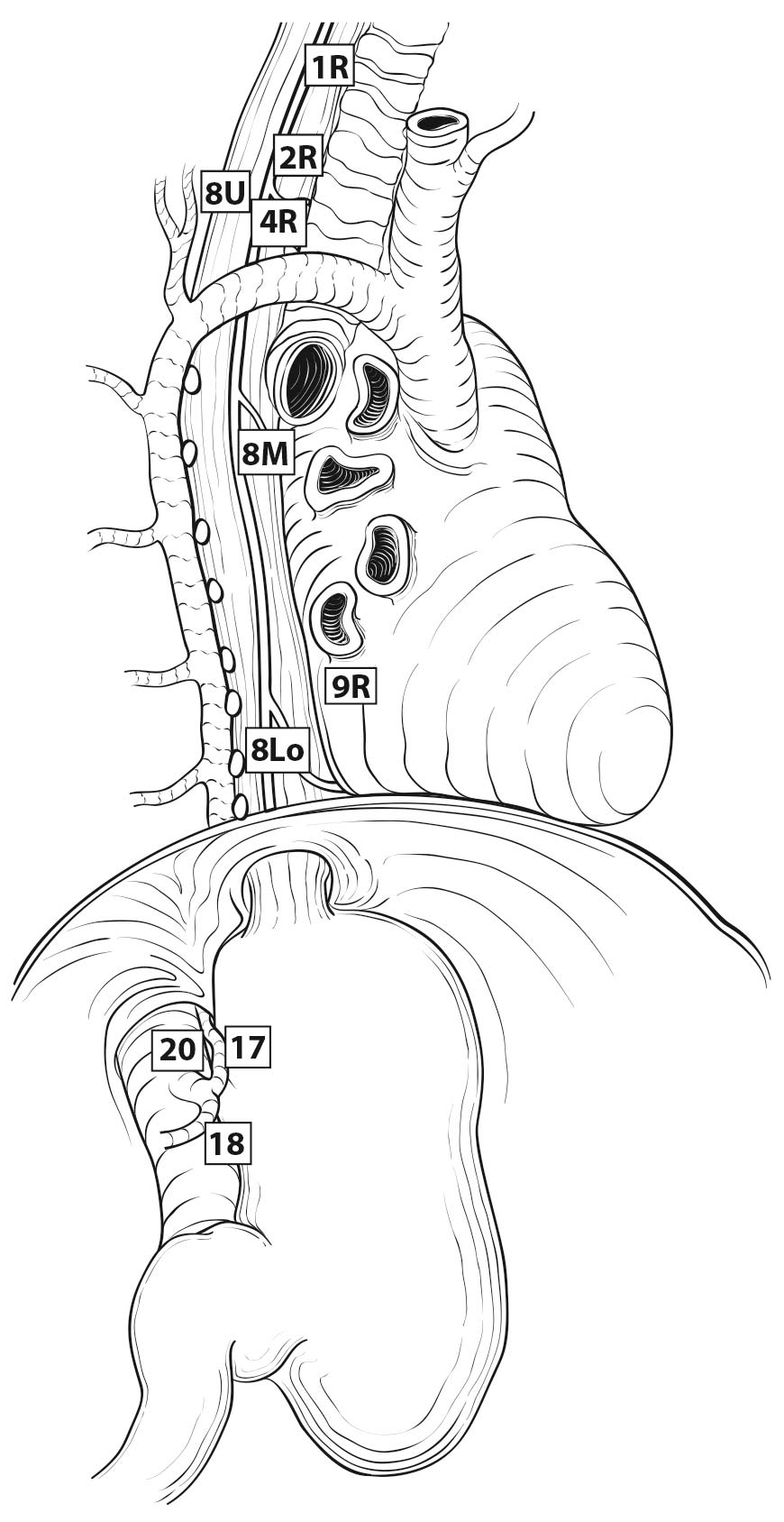
16.3_B (B) Lymph node maps for esophageal cancer. Regional lymph node stations for staging esophageal cancer from right. 1R, Right lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 1L, Left lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 2R, Right upper paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung. 2L, Left upper paratracheal nodes, between the top of the aortic arch and the apex of the lung. 4R, Right lower paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein. 4L, Left lower paratracheal nodes, between the top of the aortic arch and the carina. 7, Subcarinal nodes, caudal to the carina of the trachea. 8U, Upper thoracic paraesophageal lymph nodes, from the apex of the lung to the tracheal bifurcation. 8M, Middle thoracic paraesophageal lymph nodes, from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein. 8Lo, Lower thoracic paraesophageal lymph nodes, from the caudal margin of the inferior pulmonary vein to the EGJ. 9R, Pulmonary ligament nodes, within the right inferior pulmonary ligament. 9L, Pulmonary ligament nodes, within the left inferior pulmonary ligament. 15, Diaphragmatic nodes, lying on the dome of the diaphragm and adjacent to or behind its crura. 16, Paracardial nodes, immediately adjacent to the gastroesophageal junction. 17, Left gastric nodes, along the course of the left gastric artery. 18, Common hepatic nodes, immediately on the proximal common hepatic artery. 19, Splenic nodes, immediately on the proximal splenic artery. 20, Celiac nodes, at the base of the celiac artery.
16.3_C C) Lymph node maps for esophageal cancer. Regional lymph node stations for staging esophageal cancer, anterior view. 1R, Right lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 1L, Left lower cervical paratracheal nodes, between the supraclavicular paratracheal space and apex of the lung. 2R, Right upper paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and the apex of the lung. 2L, Left upper paratracheal nodes, between the top of the aortic arch and the apex of the lung. 4R, Right lower paratracheal nodes, between the intersection of the caudal margin of the brachiocephalic artery with the trachea and cephalic border of the azygos vein. 4L, Left lower paratracheal nodes, between the top of the aortic arch and the carina. 7, Subcarinal nodes, caudal to the carina of the trachea. 8U, Upper thoracic paraesophageal lymph nodes, from the apex of the lung to the tracheal bifurcation. 8M, Middle thoracic paraesophageal lymph nodes, from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein. 8Lo, Lower thoracic paraesophageal lymph nodes, from the caudal margin of the inferior pulmonary vein to the EGJ. 9R, Pulmonary ligament nodes, within the right inferior pulmonary ligament. 9L, Pulmonary ligament nodes, within the left inferior pulmonary ligament. 15, Diaphragmatic nodes, lying on the dome of the diaphragm and adjacent to or behind its crura. 16, Paracardial nodes, immediately adjacent to the gastroesophageal junction. 17, Left gastric nodes, along the course of the left gastric artery. 18, Common hepatic nodes, immediately on the proximal common hepatic artery. 19, Splenic nodes, immediately on the proximal splenic artery. 20, Celiac nodes, at the base of the celiac artery.