Clinical examination, abdominal/pelvic computed tomography (CT) scanning, and other appropriate imaging techniques, such as magnetic resonance (MR) imaging of the primary tumor, are required for assessment of the tumor and its extensions, both local and distant (see below). Although percutaneous biopsy may not be necessary if surgical resection is planned, it is necessary if a non-renal cell cancer is suspected (e.g., lymphoma), or if an ablative rather than surgical extirpative procedure is planned, and can be performed safely. Extensive laboratory-based workup is generally not necessary, but should include a complete blood count, basic chemistries to assess renal and liver function, and calcium and lactate dehydrogenase (LDH) levels, which may be important for prognostication, at least in metastatic disease.
Imaging
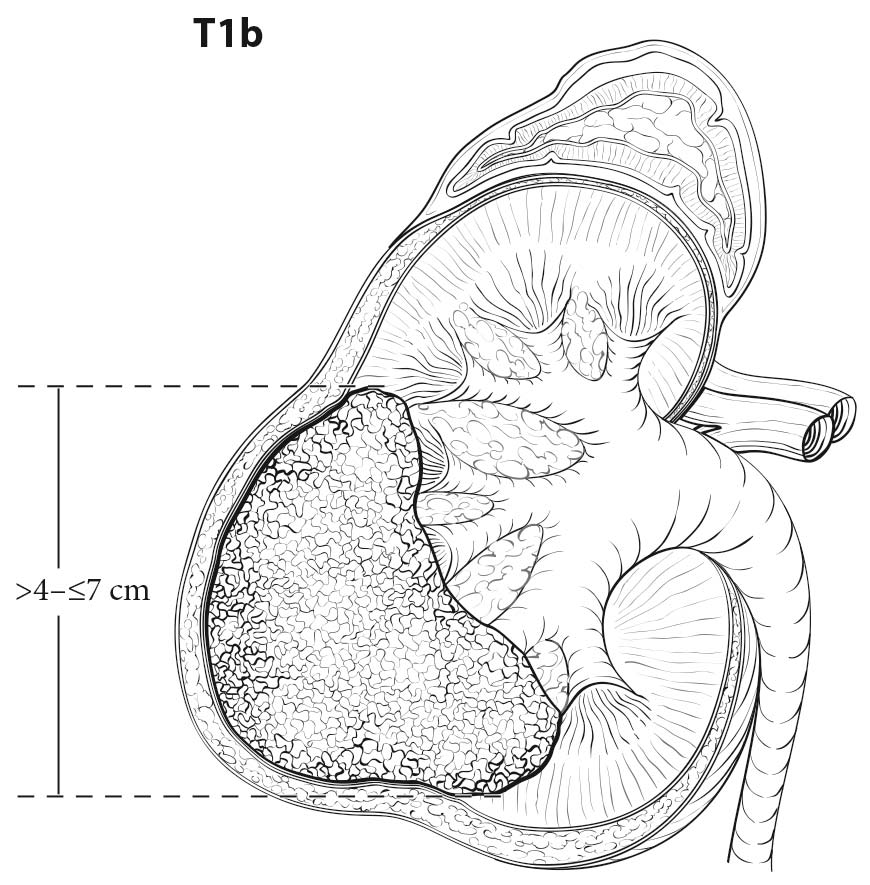
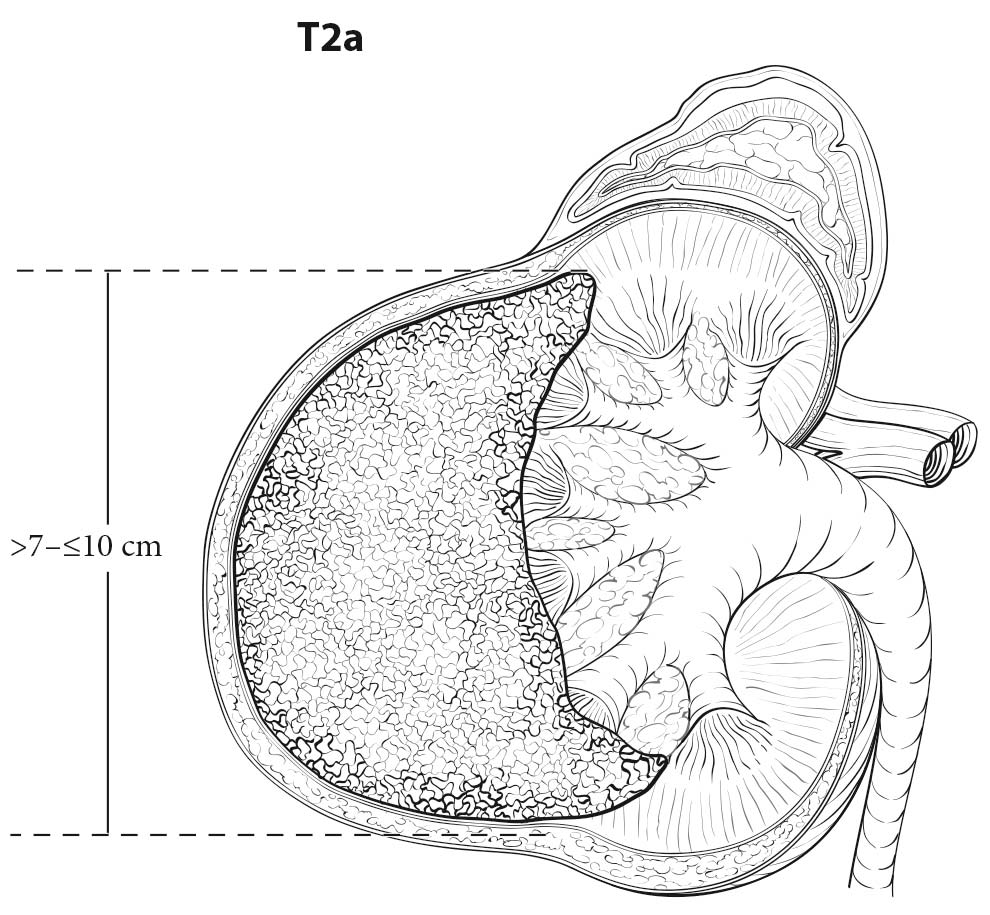
Both CT and MR imaging are equally useful in local staging of renal cell carcinoma and can be considered as first line tests. However, CT has an advantage over MR imaging as it shows calcification and allows better visualization of other body parts, such as the chest, which may be used for staging. RCC is usually a solid, contrast-enhancing mass or cystic with solid components. Most contrast-enhancing renal masses tend to be malignant, and the odds ratio of malignancy increases with increasing size. Multiplanar imaging in MR or multiplanar reconstruction of CT images allows accurate measurement of renal tumors. However, small differences between imaging size measurements and measurements made on resected tumors postoperatively are common, but may not be clinically significant.1,2 High-resolution CT using thin sections appears to improve detection of perinephric infiltration, although false positives are common.3,4
Presence of a pseudocapsule on MR imaging is useful in separating T1 or T2 tumors from T3a tumors in patients with RCC.5 Involvement of renal sinus fat is more difficult to detect on preoperative imaging. Although CT and MR imaging have a high negative predictive value, detection of renal sinus fat invasion is often difficult with relatively low positive predictive value.6
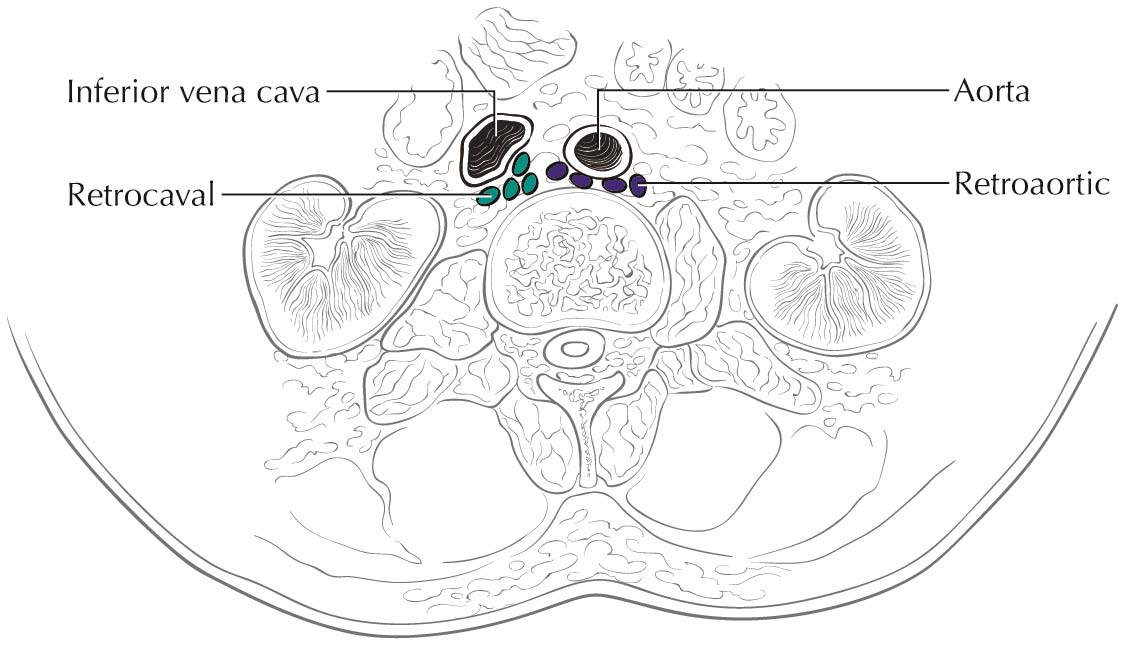
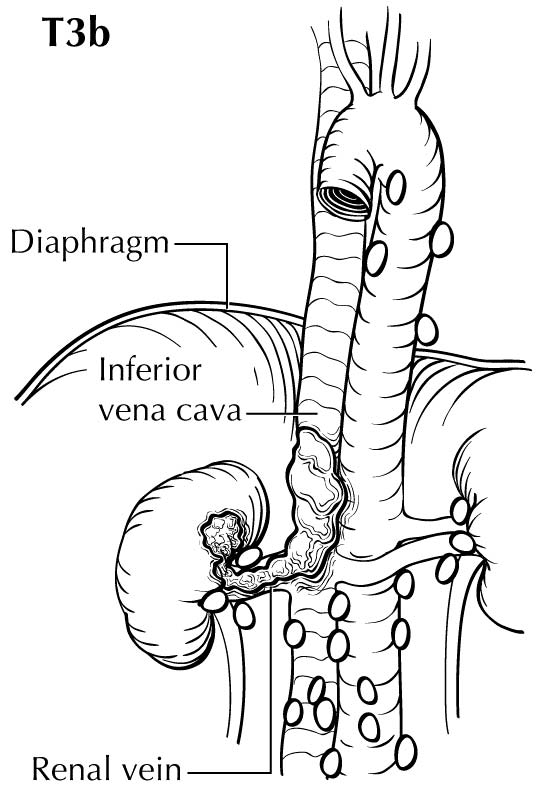
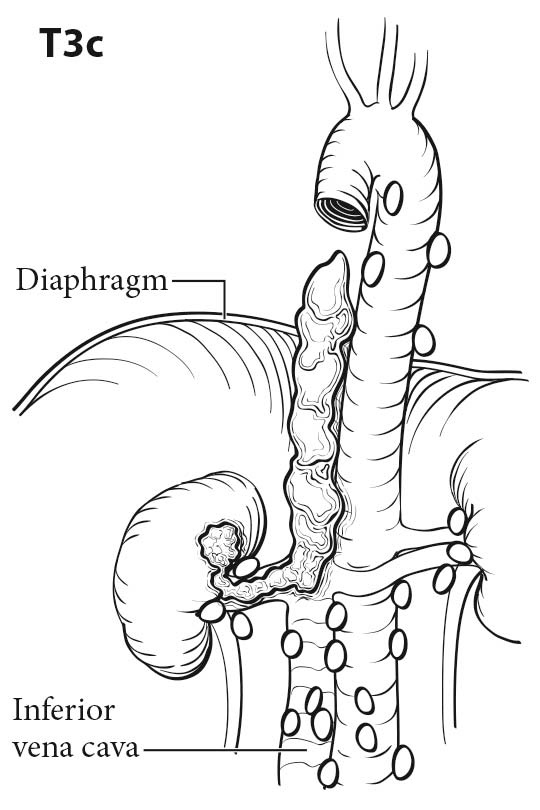
Tumor thrombus in the renal vein or inferior vena cava (IVC) can be identified on the venous or delayed phase of contrast-enhanced CT or MR imaging. Signs suggestive of renal vein or caval thrombus include filling defects, enlargement of the vessel, and rim enhancement. Tumor thrombus in the segmental branches of the renal vein may be more difficult to detect than thrombus in the main renal vein and IVC.7 Both CT and MR imaging have poor positive predictive value for distinguishing invasion from mere abutment with adjacent organs, such as adrenal gland s, liver, diaphragm, psoas muscles, pancreas, and bowel. CT and MR imaging have a high sensitivity and nearly a 100% negative predictive value in detecting direct contiguous spread to the ipsilateral adrenal gland .8,9
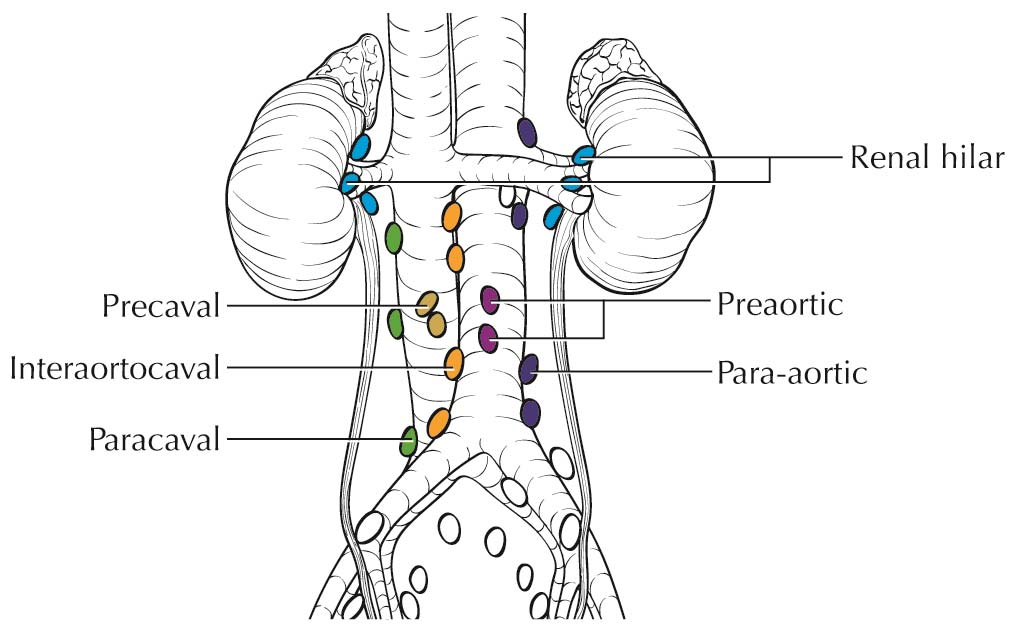
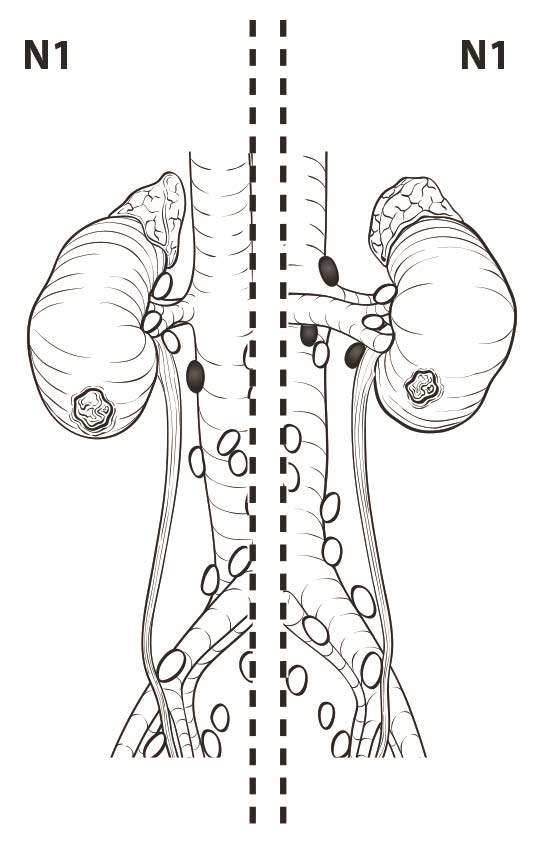
Lymph nodes larger than 1 cm in short axis diameter or nodes that appear to have distorted architecture on imaging should raise suspicion for nodal metastasis. Although 10% of nodes that harbor metastases may be smaller than 1 cm, reactive hyperplasia is common and can be seen in up to 58% of enlarged nodes.10
The risk of metastasis depends on the size of renal tumors and other factors, such as subtype and sarcomatoid differentiation.11-14 Distant metastases typically occur in lung, bone, liver, ipsilateral and contralateral adrenal gland s, and brain. Chest CT is the most sensitive test for detecting pulmonary metastasis, but a plain chest X-ray may be sufficient in low-risk patients.12,15 Patients with advanced primary tumors with symptoms attributable to bone metastasis or patients with abnormal laboratory findings, such as elevated alkaline phosphatase, can be investigated with bone scan to detect bone metastasis.16 Patients with localizing neurological signs should be investigated with MR imaging of the brain or a contrast-enhanced CT scan of the head to detect brain metastasis. Although no evidence justifies routine use of brain MR imaging, it can be used to detect asymptomatic occult brain metastasis in patients with advanced RCC.17 Combined 18-F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET-CT or PETCT) does not have an established role in the initial staging of renal cancer, in part because RCC lesions typically have low avidity for FDG relative to high background uptake and excretion in the kidneys.18 Although low in sensitivity to detect distant metastasis, PET-CT has superior specificity and may have a complementary role as a problem-solving tool in cases that are equivocal by conventional imaging.19,20