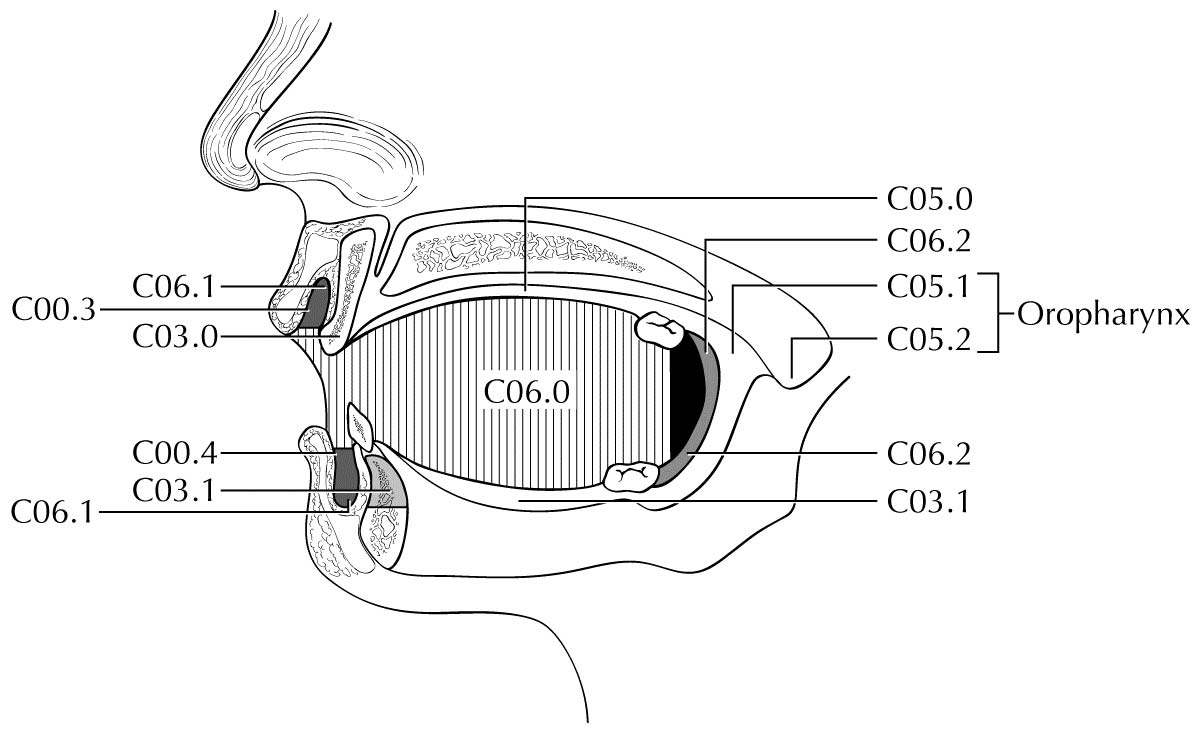
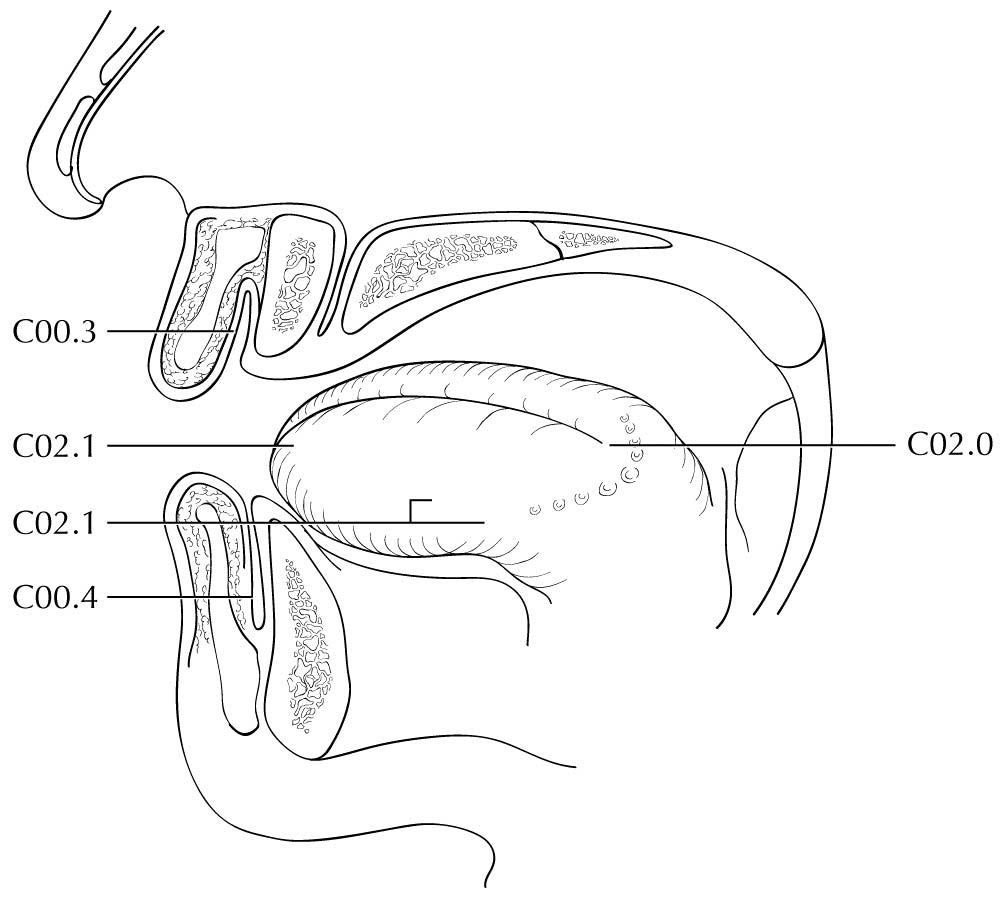
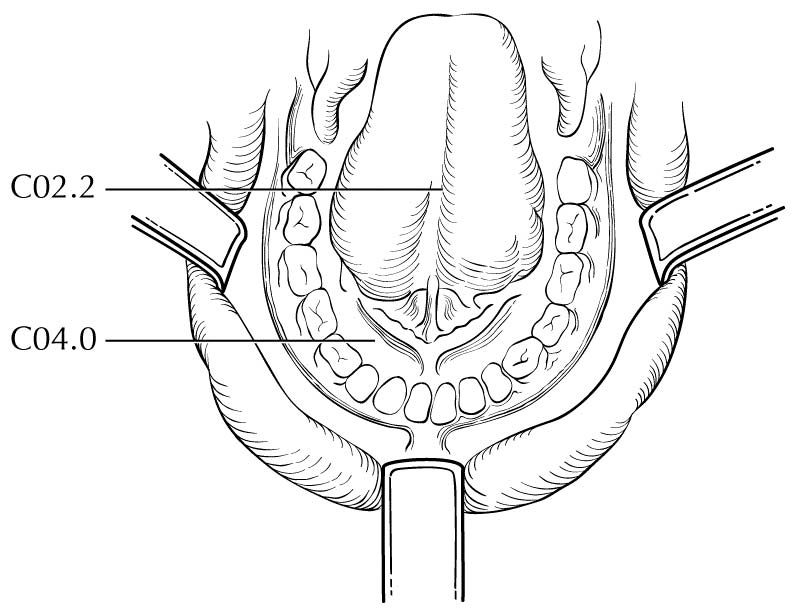
Clinical Classification
Clinical staging for Lip and Oral Cavity cancers is predicated most strongly upon the history and physical examination. Biopsy is necessary to confirm diagnosis and is typically done of the primary. Nodal biopsy is done by fine needle aspiration when indicated. Results from diagnostic biopsy of the primary tumor, regional nodes, and distant metastases can be included in clinical classification.
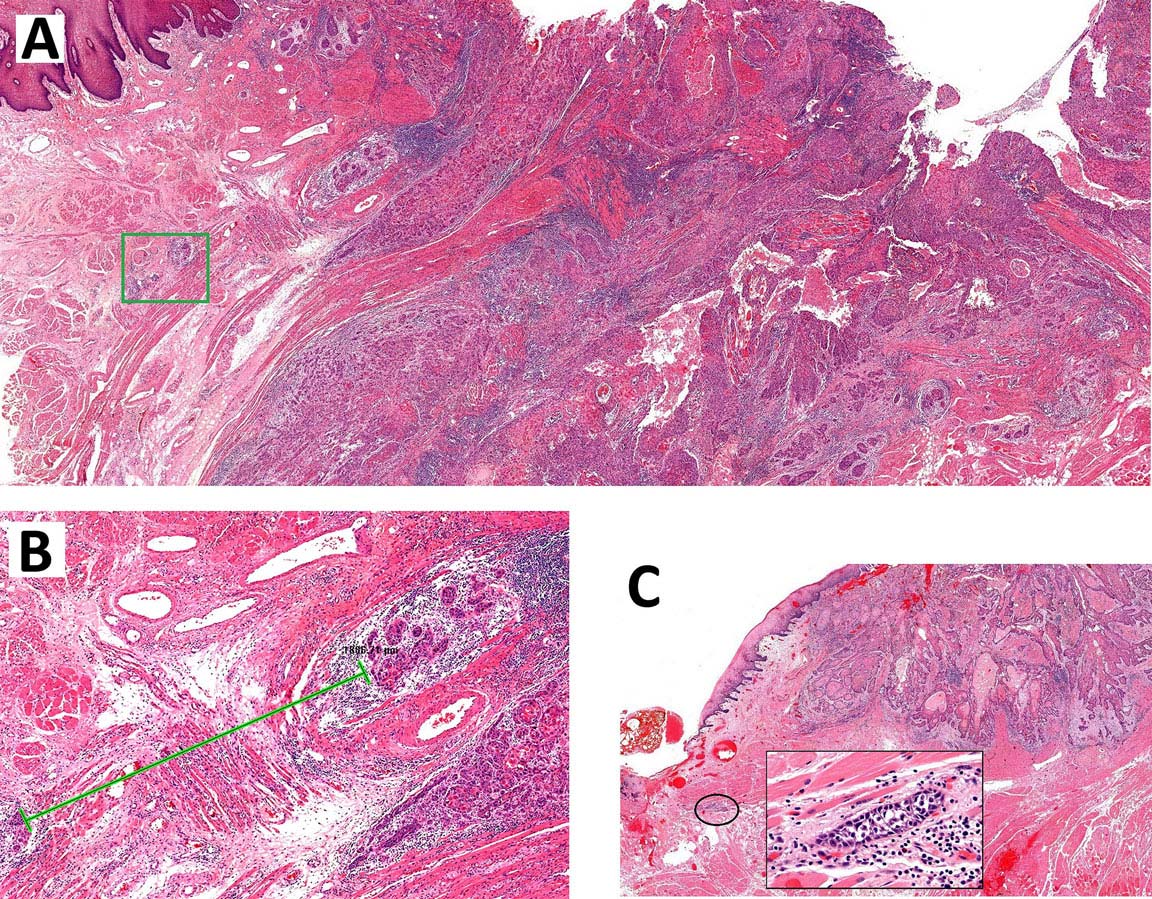
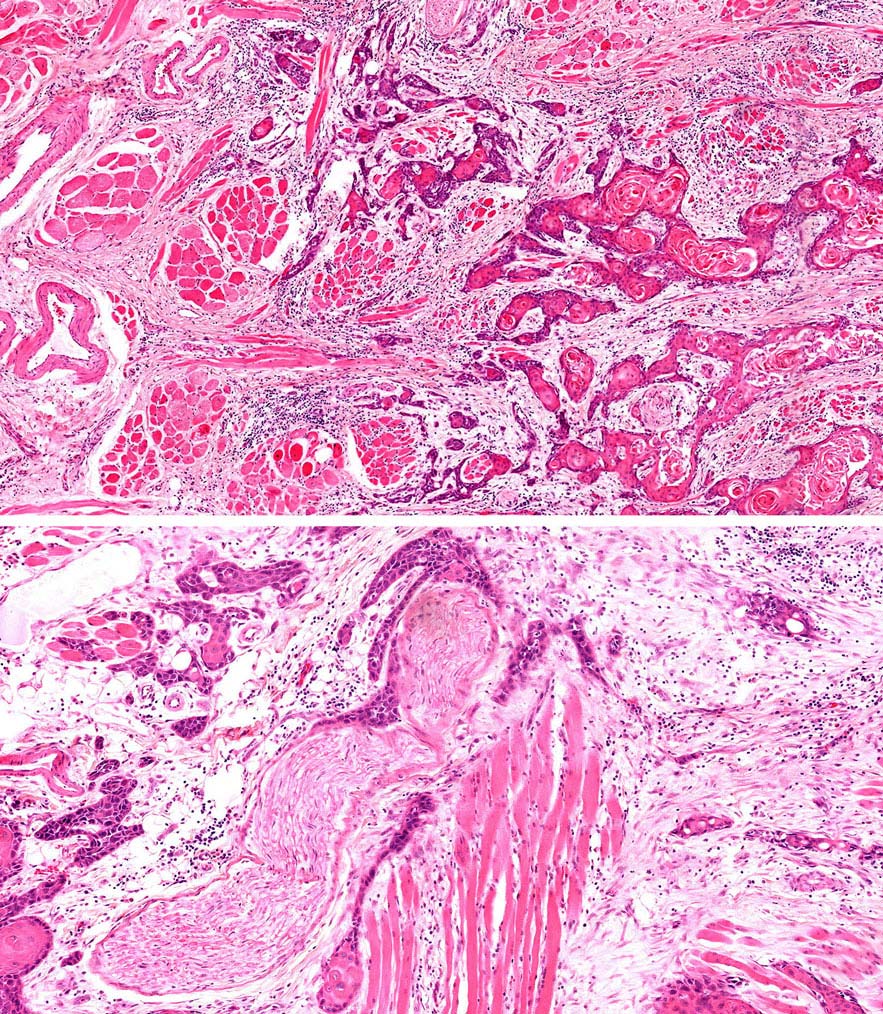
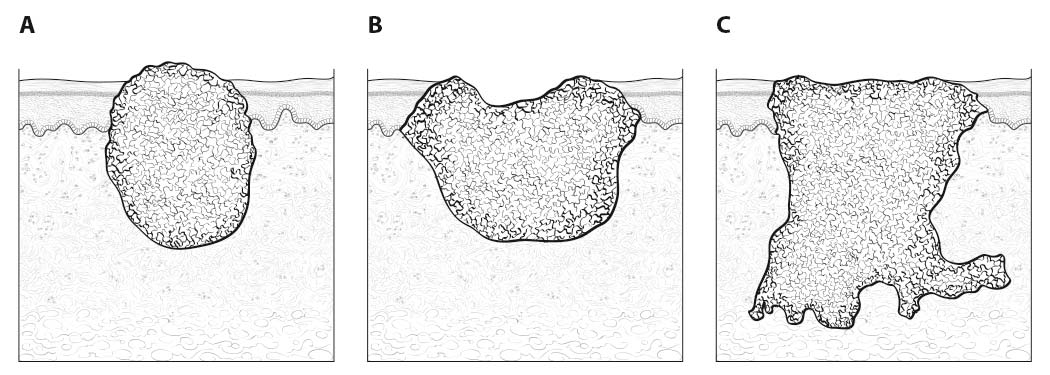
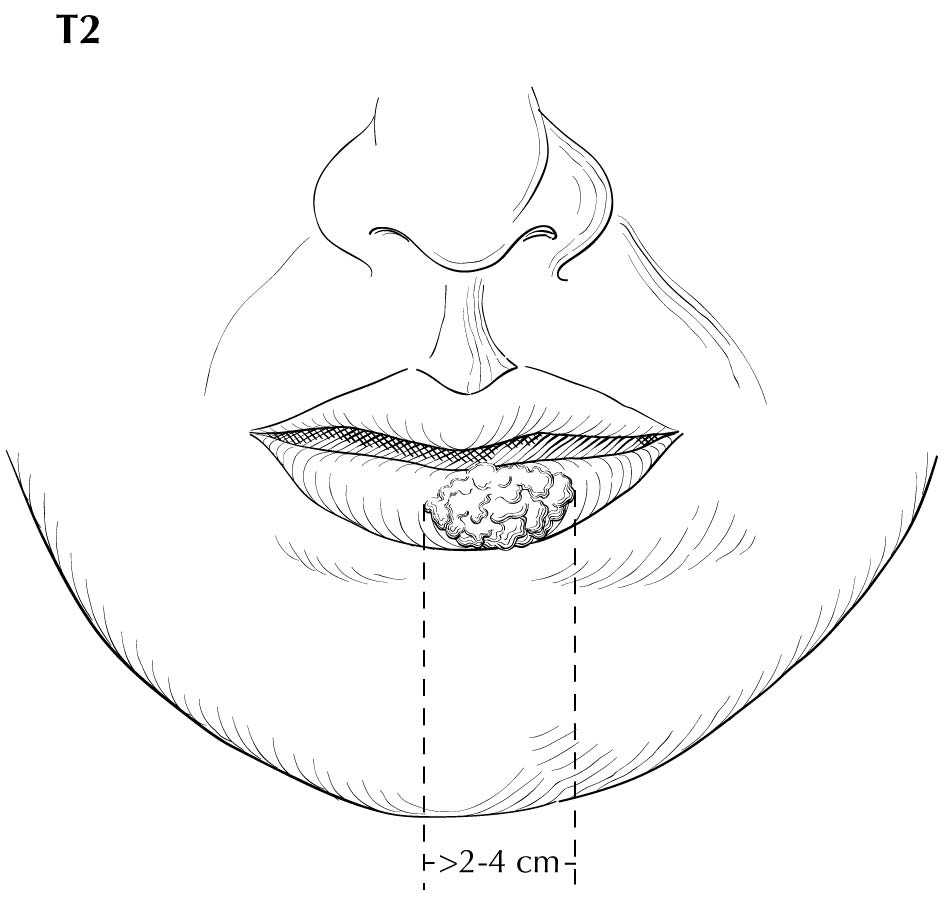
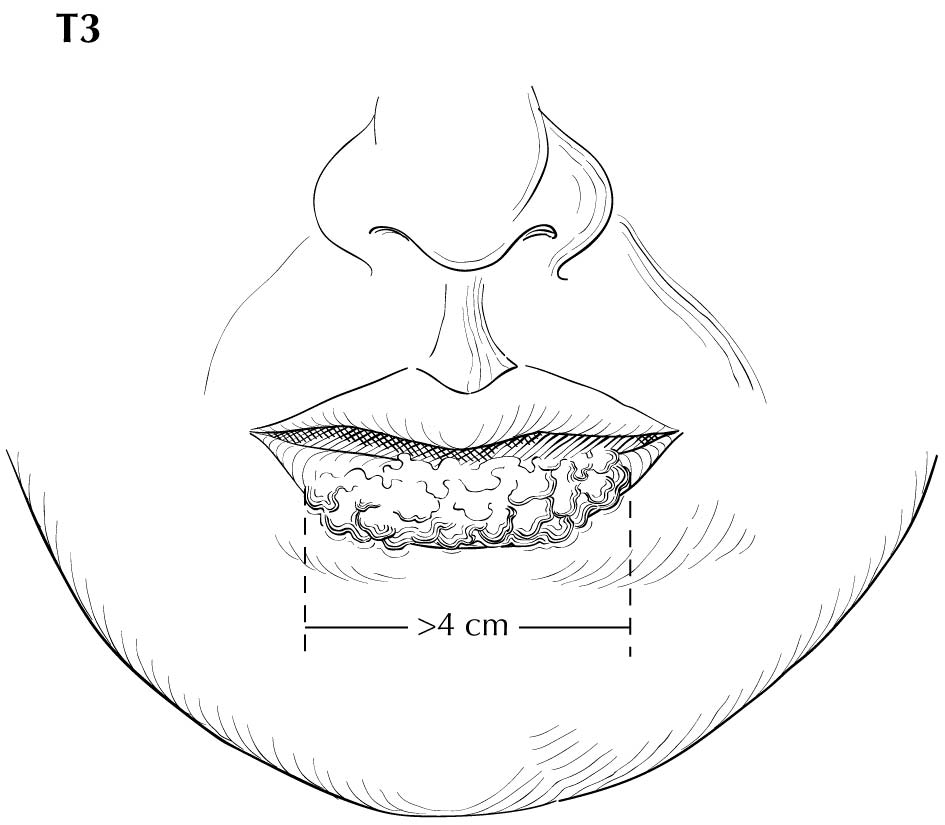
Inspection of the lip and oral cavity typically reveals the greatest diameter of a cancer, though palpation is essential to assess DOI and submucosal extension. The mucosal extent of the cancer usually reflects its true linear dimension. Induration surrounding a cancer typically is due to peritumoral inflammation. DOI should be distinguished from tumor thickness, and its determination is predicated on invasion beneath the plane defined by surrounding normal mucosa. Any exophytic character should be noted, but assignment of stage is determined by what transpires at or beneath the surface (defined by adjacent normal mucosa). Clinical evidence of bone destruction should be noted and its depth estimated (e.g., into bone versus through cortex into the marrow space). Thick lesions often are defined by computed tomography (CT) or magnetic resonance (MR) imaging, but the difference between thickness and DOI must be observed. Lesions located near the midline more often involve the contralateral side of the neck than well-lateralized cancers. Dysphagia is suggestive of a tumor with sufficient invasion of oral structures to engender dysfunction. It is seldom present when cancers have little DOI. Similarly, drooling or the inability to swallow liquids without difficulty suggests a tumor with substantial DOI. Trismus, when not caused by pain, is consistent with a deeply invasive lesion. Complaints of numbness of the lip and /or teeth are commonly associated with nerve invasion. The distinction between 4 mm DOI and 6 mm DOI (for example) may not be possible on clinical grounds. The stage should only be raised on the basis of DOI if the differences are clear.
Evidence of cranial nerve dysfunction should be sought (testing sensation and motion to command ) and skin should be examined for evidence of invasion by underlying nodes. Palpable neck nodes should be considered in terms of their location (level in the neck), size, number, character (smooth or irregular), attachment to other nodes, and mobility. Nodes that do not move in all directions may be invading nearby structures. Invasion of the sternomastoid muscle and /or cranial nerves is associated with lateral motion with restricted ability to move the node along the cranial-caudal axis. Inability to move the node at all (without moving the head) is worrisome for ENE, though the suspicion should be tempered for smaller nodes with limited mobility in level II. Assignment of ENE should be based almost entirely upon the physical examination, rather than upon imaging studies; gross ENE is required to raise the stage beyond the assignment based upon node size and number, and this may be overestimated with current imaging modalities.
Clinical or radiographic extranodal extension
ENE worsens the adverse outcome associated with nodal metastasis. The presence of ENE can be diagnosed clinically by the presence of a “matted” mass of nodes, involvement of overlying skin, adjacent soft tissue, or clinical signs of cranial nerve or brachial plexus, sympathetic chain or phrenic nerve invasion. Cross-sectional imaging (CT or MR) generally has low sensitivity (65-80%) but high specificity (86-93%) for the detection of ENE. The most reliable imaging signs are an indistinct nodal margin, irregular nodal capsular enhancement or infiltration into the adjacent fat or muscle, with the latter finding on CT and MR imaging as the most specific sign of ENE. Ultrasound appears to be less accurate than CT and MR imaging, but ENE is suggested by interrupted or undefined nodal contours with high-resolution ultrasound imaging. The absence or presence of clinical/radiologic ENE is designated ENE(-) or ENE(+), respectively.
Imaging
Cross-sectional imaging of the oral cavity may be performed with either CT or MR imaging, depending on availability, patient imaging tolerance, contrast allergies, and cost. With either modality, the coronal plane view—either as direct MR imaging or from reformats obtained from axially acquired thin-slice CT—allows excellent evaluation of the floor of the mouth.9 CT offers some advantage over MR imaging in the evaluation of cortical bone erosion, although MR imaging appears to be more sensitive but less specific for the detection of bone marrow invasion by tumor.10,11 MR imaging offers the additional advantage of evaluation of perineural tumor spread, which for oral cavity tumors is primarily along the inferior alveolar nerve (CNV3) of the mand ible and the greater and lesser palatine nerves (CNV2) of the maxilla. Gadolinium contrast is always recommended unless contraindicated by prior reaction or very poor renal function. Positron emission tomography (PET)/CT is primarily done for nodal staging of disease or when distant metastases are suspected, unless the CT component is performed as a post-contrast examination with dedicated neck imaging. Ultrasound does not allow adequate evaluation of the oral cavity primary tumor site, but it may be supplementary for nodal evaluation with otherwise equivocal nodal imaging findings.
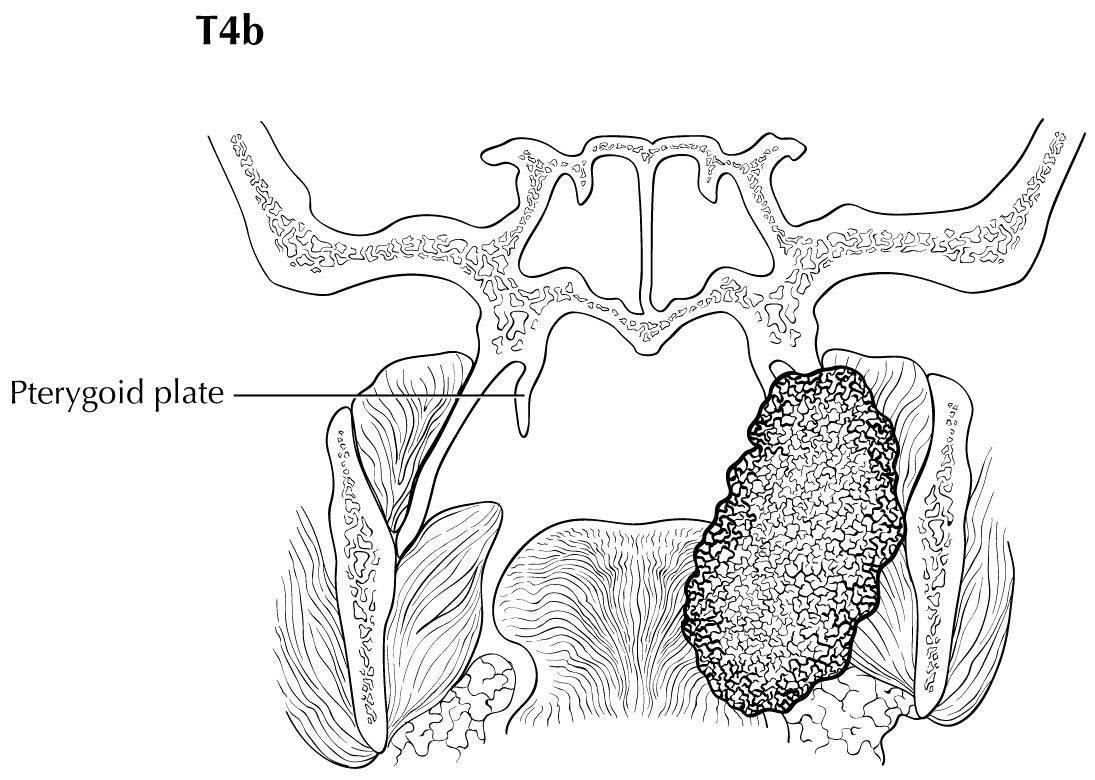
As small but clinically evident mucosal tumors may be subtle on imaging, it is important to review the imaging exam with knowledge of the tumor site. T1, T2, and T3 tumors are distinguished only by size and depth of invasion. The former is better determined by clinical examination, although a radiologic measurement should be given as part of the imaging report. The radiologist's more important role during tumor staging is to determine deep tissue involvement and assess for nodal and /or distant metastases. T4 disease entails deep tissue invasion, which varies according to the specific subsite of the oral cavity. For alveolar ridge, floor of mouth, retromolar triangle, hard palate, and large lip tumors, careful attention should be paid to the cortex and marrow space of the adjacent maxilla or mand ible, because such invasion denotes T4a disease. In the AJCC Cancer Staging Manual, 7th Edition, oral tongue tumors were designated T4a when there was deep invasion into the extrinsic muscles of the tongue and /or the floor of the mouth. DOI will supersede muscle invasion in the 8th Edition. Depth is frequently better evaluated in the coronal plane and /or sagittal plane. More posterior extensive spread of tumor—such as buccal tumors invading into the muscles of mastication, or spreading to the pterygoid plates or superiorly to the skull base—denotes T4b tumor. Additionally, posterolateral tumor spread to surround the internal carotid artery is also T4b disease.
Both CT and MR imaging allow evaluation of nodal morphology to determine possible tumor involvement. Levels IA, IB, and IIA are the most frequently involved sites, and these levels should be scrutinized specifically with concern for rounded contour, heterogeneous texture including cystic or necrotic change, enlargement, and ill-defined margins. It also is important to be cognizant that nodal spread may be bilateral, particularly with anterior and /or midline oral cavity tumors. Skip nodal metastases (level IV without level III involvement) while described with lateral tongue tumors, appear to be rare. As previously described, PET/CT may also be used to improve predictive yield for nodal metastases by the addition of physiologic information, and ultrasound may be an additive tool for evaluation of indeterminate nodes. PET/CT is the only modality to allow whole-body evaluation of distant metastatic spread, and the upper lungs and bone should always be reviewed as potential metastatic sites on any staging neck CT or MR imaging.
The risk of distant metastasis is more dependent on the N than on the T status of the head and neck cancer. In addition to the node size, number, and presence of ENE, regional lymph nodes also should be described according to the level of the neck that is involved. The level of involved nodes in the neck is prognostically significant for oral cavity (caudad nodal disease is worse), as is the presence of ENE metastatic tumor from individual nodes. Midline nodes are considered ipsilateral. Imaging studies showing amorphous spiculated margins of involved nodes or involvement of internodal fat resulting in loss of normal oval-to-round nodal shape strongly suggest extranodal extension; however, pathological examination is necessary to prove its presence. No imaging study can currently identify microscopic foci of cancer in regional nodes or distinguish between small reactive nodes and small nodes with metastatic deposits (in the absence of central radiographic inhomogeneity).
Pathological Classification
Complete resection of the primary site and /or regional lymph node dissections, followed by pathological examination of the resection specimen allows for the use of this designation for pT and /or pN, respectively. Resections after radiation or chemotherapy should be identified and considered in context. pT is derived from the actual measurement of the unfixed tumor in the surgical specimen. It should be noted, however, that up to 30% shrinkage of soft tissues may occur in resected specimen after formalin fixation. Pathological staging represents additional and important information and should be included as such in staging, but it does not supplant clinical staging as the primary staging scheme. Metastasis found on imaging is considered cM1. Biopsy-proven metastasis is considered pM1.
Pathological assessment of ENE
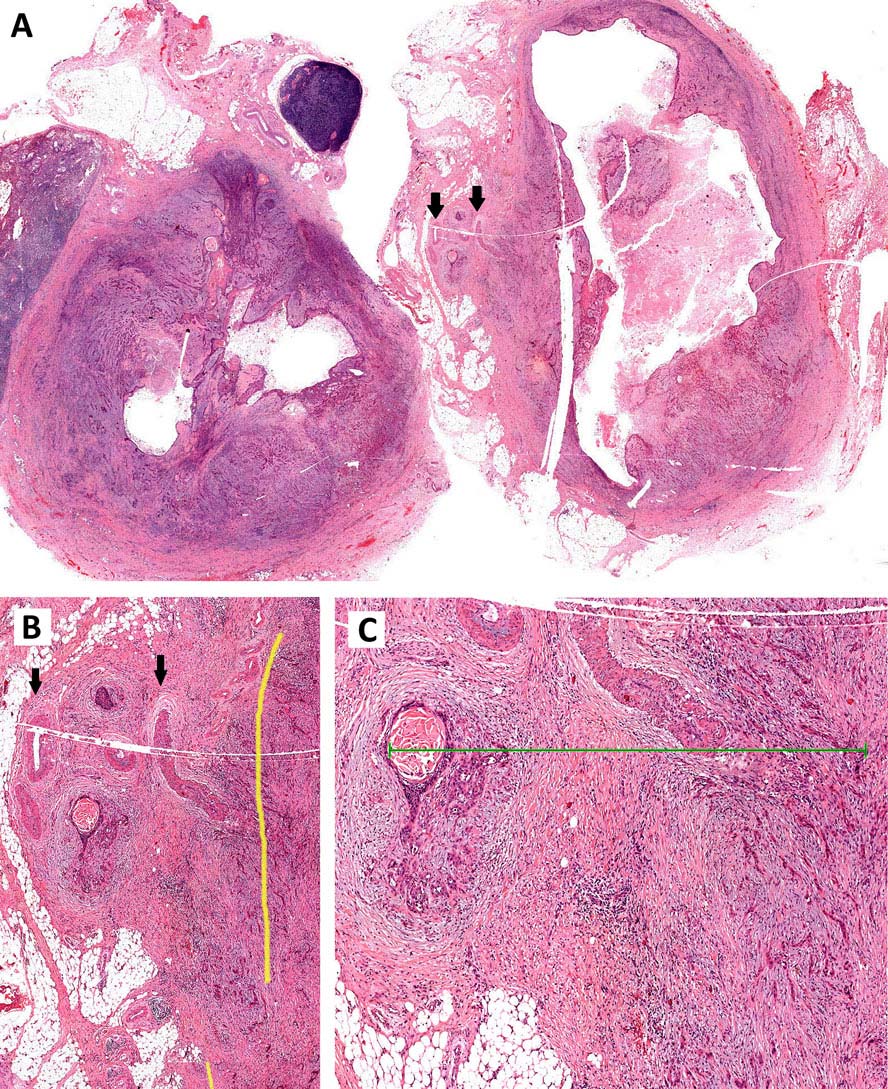
Resected positive lymph nodes require examination for the presence and extent of ENE. ENEmi is defined as microscopic ENE ≤ 2 mm. Macroscopic (ENEma) is defined as either extranodal extension apparent to the naked eye at the time of dissection or extension > 2 mm beyond the lymph node capsule microscopically. ENEmi and ENEma are used to define pathological ENE(+) nodal status.
For assessment of pN, a selective neck dissection will ordinarily include 10 or more lymph nodes, and a comprehensive neck dissection (radical or modified radical neck dissection) will ordinarily include 15 or more lymph nodes. Examination of fewer tumor-free nodes still mand ates a pN0 designation.