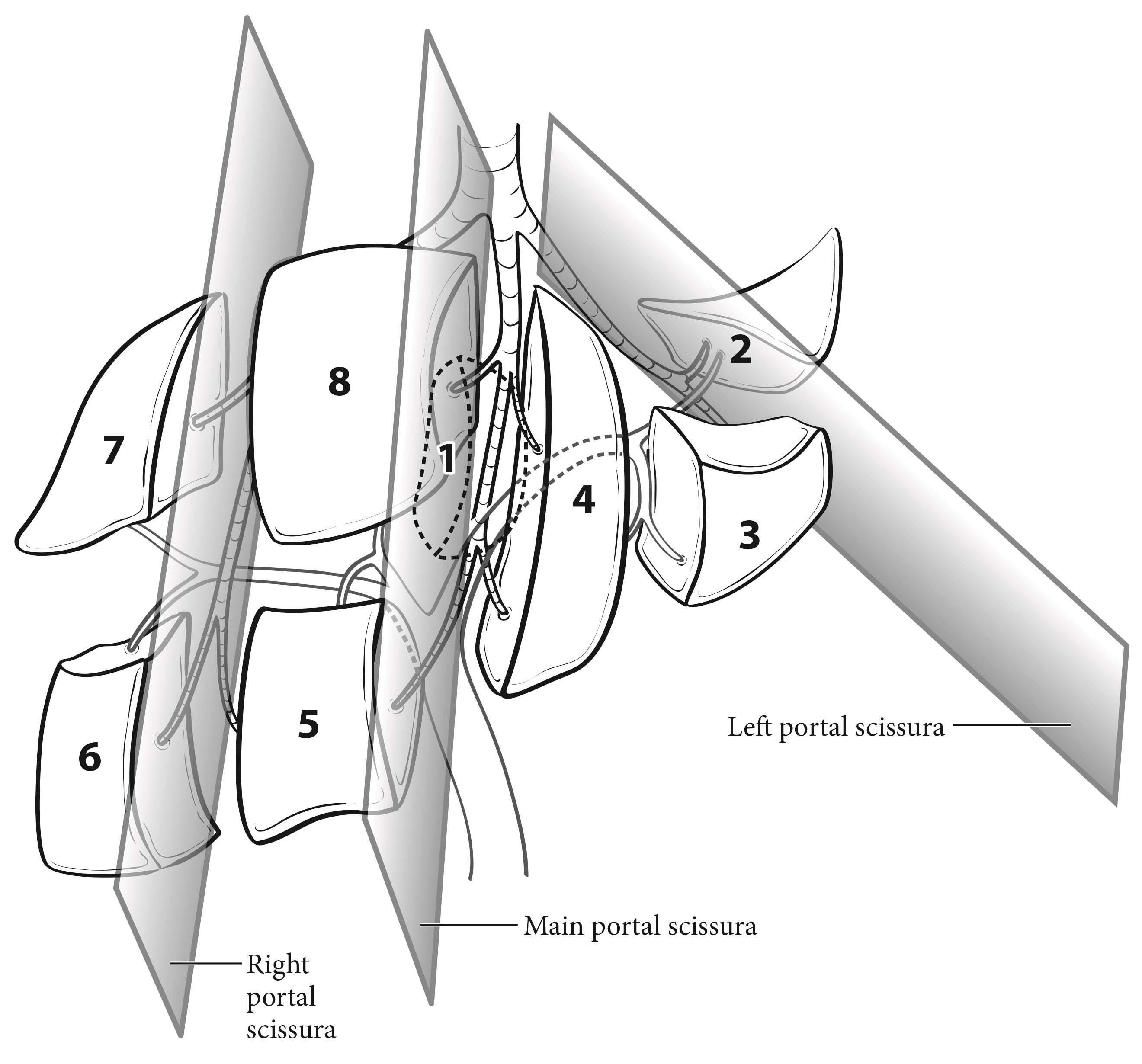
The liver has a dual blood supply from the hepatic artery and portal vein. Tumors are fed by the arterial blood supply. The liver is divided into right and left hemilivers by a plane called the Rex-Cantlie line, which projects between the gallbladder fossa and the vena cava and is defined by the middle hepatic vein. Couinaud refined knowledge about the functional anatomy of the liver and proposed dividing the liver into four sectors and eight segments. In this nomenclature, the liver is divided by vertical and oblique planes, or scissurae, defined by the three main hepatic veins, and a transverse plane or scissura that follows a line drawn through the right and left portal branches, making the four sectors (right paramedian, right lateral, left paramedian, and left lateral), which are further divided into segments by the transverse scissura (Figure 30.1). The eight segments are numbered clockwise in the frontal plane. Recent advances in hepatic surgery have enabled anatomic (also called systematic) resections along these planes.
Histologically, the liver is divided into lobules, each of which is drained by central veins. The portal triads between the lobules contain the intrahepatic bile ducts and the blood supply, which consists of small branches of the hepatic artery and portal vein and intrahepatic lymphatic channels.
HCC may spread through capsular invasion, extracapsular invasion, vascular invasion, and/or intrahepatic metastases. Tumors may extend through the liver capsule to adjacent organs (adrenal gland, diaphragm, and colon) or may rupture, causing acute hemorrhage and peritoneal metastasis.
30.1 Couinaud's segmental anatomy of the liver. The liver is divided into two hemilivers and eight segments according to the portal venous ramification pattern. Three major hepatic veins represent the position of scissural planes.
The regional lymph nodes are the hilar, hepatoduodenal ligament, inferior phrenic, and caval lymph nodes, among which the most prominent are the hepatic artery and portal vein lymph nodes.
Clinical manifestations may include malaise, anorexia, and abdominal pain. A mass effect or cirrhosis-related ascites may cause abdominal fullness. Spontaneous rupture, causing acute abdominal pain and distension, represents a potentially fatal event that warrants prompt diagnosis and management. Hepatitis serologic studies—hepatitis B surface antigen, hepatitis B core antibody, and hepatitis C antibody—are warranted. If applicable, a polymerase chain reaction quantitative viral load assay also should be performed. An assessment of liver function and degree of cirrhosis is key; the Child-Pugh scoring system is used most commonly (Table 30.1). In patients treated with systemic therapy, liver biopsy is important for translational research to elucidate key signaling pathways that may be targeted with novel therapies. Liver biopsy is a comparatively safe and well-tolerated procedure.
30.1 Child-Pugh Score
| Points | |||
| 1 | 2 | 3 | |
| Albumin (g/dL) | >3.5 | 2.8-3.5 | <2.8 |
| Bilirubin (mg/dL) | <2.0 | 2.0-3.0 | >3.0 |
| Prothrombin time | |||
| Seconds | <4 | 4-6 | >6 |
| INR | <1.7 | 1.7-2.3 | >2.3 |
| Ascites | None | Moderate | Severe |
| Encephalopathy | None | Grade I-II | Grade III-VI |
| Child-Pugh class A | 5-6 points | ||
| Child-Pugh class B | 7-9 points | ||
| Child-Pugh class C | 10-15 points | ||
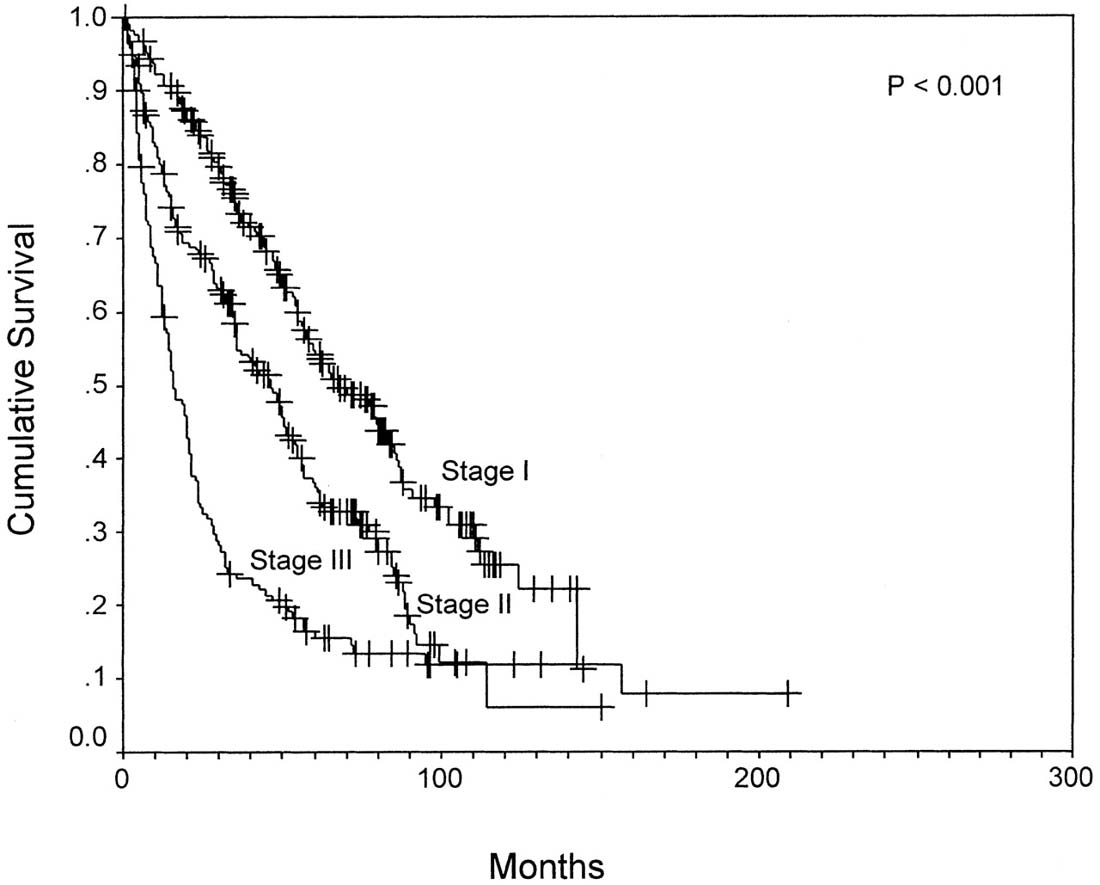
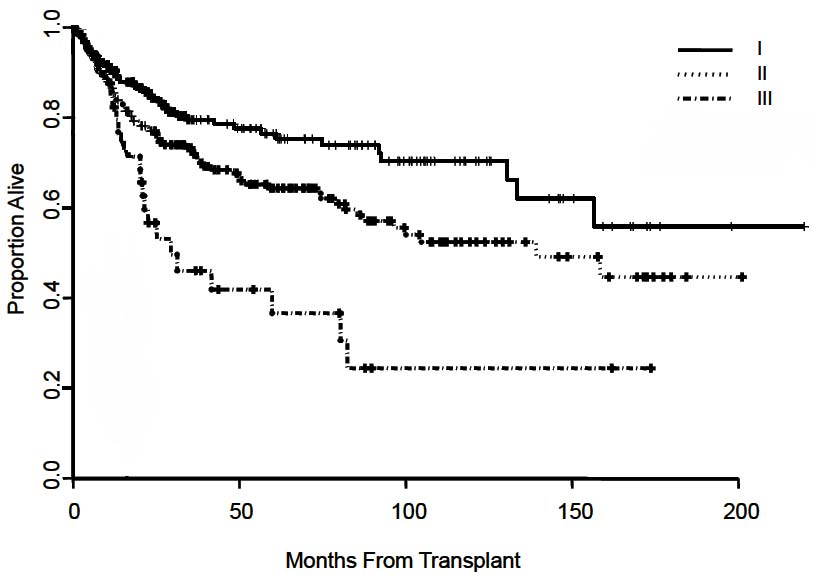
The T classification is based primarily on the results of a multicenter international study of pathological factors affecting prognosis after resection of HCC.3 The classification considers the presence or absence of vascular invasion (as assessed radiographically or microscopically), the number of tumor nodules (single vs. multiple), and the size of the largest tumor. The simplified classification adopted in the AJCC Cancer Staging Manual, 6th Edition and 7th Edition, stratifies patient survival well (Figure 30.2). This staging system subsequently was validated in multiple studies after liver resection4-10 and in a large multicenter series after liver transplantation (Figure 30.3).11
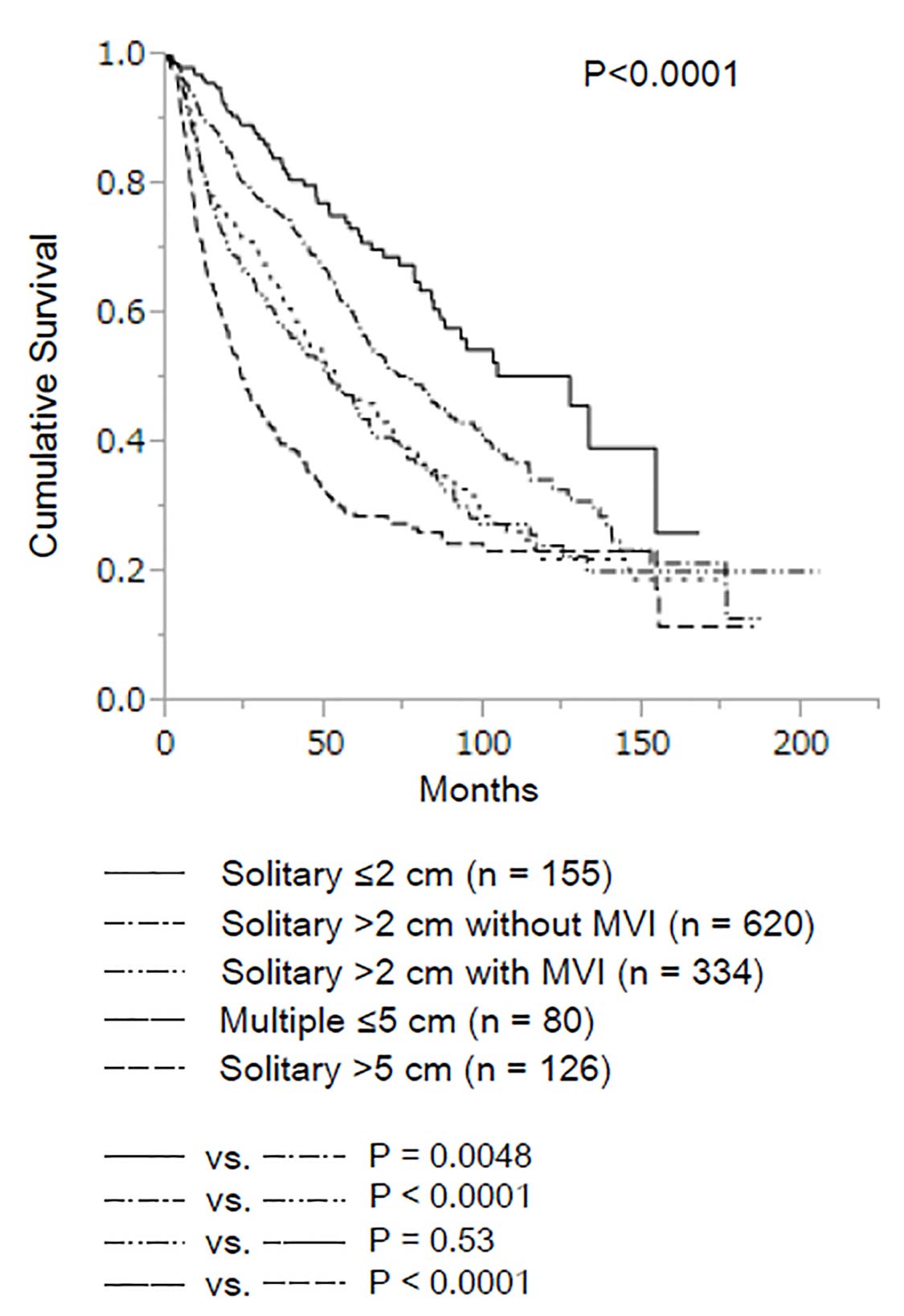
In a recent study of 1,109 patients with solitary HCC measuring up to 2 cm, neither microvascular invasion nor histologic grade had an impact on long-term survival (Figure 30.4).12 Based on these data, the AJCC Cancer Staging Manual, 8th Edition divides T1 disease into two subcategories: T1a, for patients with solitary HCC less than or equal to 2 cm irrespective of microvascular invasion, and T1b for patients with solitary HCC greater than 2 cm without microvascular invasion. The survival curve for solitary HCC greater than 2 cm with microvascular invasion was similar to that for multiple HCCs less than or equal to 5 cm. Therefore, these two groups were classified together in a revised T2 category.
In another long-term survival study of 754 patients, there was no survival difference between patients with T3a and those with T3b tumors (p = 0.073), or between patients with T3b and those with T4 tumors (p = 0.227).13 Thus, the revised 8th Edition reclassifies T3a as T3 and adds T3b to the T4 category.
Major vascular invasion is defined as invasion of the branches of the main portal vein (right or left portal vein, excluding the sectoral and segmental branches),3 one or more of the three hepatic veins (right, middle, or left),3 or the main branches of the proper hepatic artery (right or left hepatic artery).
Multiple tumors include satellitosis, multifocal tumors, and intrahepatic metastases. Assessment of lymph node involvement by clinical or radiographic means is a challenge, as reactive lymph nodes may be present. Invasion of adjacent organs other than the gallbladder or perforation of the visceral peritoneum is considered T4.
Several imaging modalities have relatively high sensitivity and specificity for diagnosis or staging of HCC, although test performance is suboptimal for small or well-differentiated HCC. Computed tomography (CT) and magnetic resonance (MR) imaging with intravenous contrast are the preferred examinations to detect HCC, and constitute key elements in defining the TNM stage.14-16 CT scanning should be performed with hepatic arterial, portal venous, and delayed venous phases. Similarly, if MR imaging is used, precontrast, arterial, venous, and delayed phases are essential. CT scanning frequently is the first examination, particularly if MR imaging is not available or is contraindicated. Ultrasound has lower sensitivity for detection of HCC, although it may be used to evaluate for vascular invasion of the portal and hepatic veins through color Doppler imaging.
Suggested Report Format- Liver morphology: describe whether cirrhotic or noncirrhotic
- Portal hypertension: spleen size, ascites, varices
- Tumor
- Primary tumor
- Number
- Size (centimeters)
- Location: involved segments
- Characterization (enhancement, pseudocapsule, fat on in- and opposed-phase T1-weighted MR imaging, calcification)
- Satellite lesion(s)
- Local extent
- If present, describe vascular involvement.
- Regional lymph nodes
- If present, describe abnormal or suspicious nodes, especially those in the porta hepatis, periceliac, and portacaval spaces.
- Distant metastases
- If present, describe metastatic lesions on CT, MR imaging, PET/CT, or bone scans.
Complete pathological staging consists of evaluation of the primary tumor, including histologic grade, regional lymph node status, and underlying liver disease. Tumor size, number, and margin add to the critical prognostic data. Portal venous tumor thrombus should be clearly documented, as it carries a poor prognosis. Tumor grade is based on the degree of nuclear pleomorphism, as described by Edmonson and Steiner. Because of the prognostic significance of underlying liver disease in HCC, it is recommended that the results of the histopathologic analysis of the adjacent (nontumorous) liver be reported. Advanced fibrosis/cirrhosis (modified Ishak score of 5-6) is associated with a worse prognosis than absence of or moderate fibrosis (modified Ishak score of 0-4). Although grade and underlying liver disease have prognostic significance, they are not included in the current staging system.
Regional lymph node involvement is rare (5%). Positive lymph nodes are classified as Stage IV because they carry the same prognosis as cases with distant metastases. For pathological classification, vascular invasion includes gross as well as microscopic involvement of vessels.
- AFP
- Fibrosis score
- Hepatitis serology
- Creatinine (part of the MELD score)
- Bilirubin (part of the MELD score)
- Prothrombin time (INR; part of the MELD score)