This chapter addresses malignant melanoma of the uvea, the most common intraocular cancer in adults. It predominantly affects white Caucasians and Hispanic people, as compared with Asian and African populations. It is a cancer that shows a high propensity to metastasize hematogenously to the liver. Staging of uveal melanoma is divided in two systems, one for anteriorly located iris melanomas and the other for posteriorly located ciliary body and choroidal melanomas, because these two types differ not only in anatomic location but also in prognosis. Both systems are based on assessment of the anatomic extent of the tumor. The system for ciliary body and choroidal melanoma was revised extensively for the AJCC Cancer Staging Manual, 7th Edition; it is evidence-based and has since been externally validated. Only minor adjustments are introduced in the AJCC Cancer Staging Manual, 8th Edition. Most uveal melanomas are managed conservatively, mainly with radiotherapy, although select large tumors continue to be treated with enucleation.1 Prognostic biopsies of conservatively treated uveal melanomas that allow analysis of their cytogenetic, gene expression, and molecular genetic features are increasingly common. However, evidence for a long-term association between these characteristics and survival according to the anatomic extent of the tumor is still incomplete.
Uveal melanoma may occur in the iris, ciliary body, or choroid. Of these sites, choroidal melanoma is the most common, with an estimated 8,000 new cases per year. The original AJCC Cancer Staging Manual, 2nd to 5th Edition staging systems for uveal melanoma was based on definitions from one epidemiological study.2 In 2003, in an effort to be relevant to the size categories widely in use at that time, the AJCC Cancer Staging Manual, 6th Edition staging was reconciled with that of the Collaborative Ocular Melanoma Study (COMS).2 For these editions, there was no foundation of clinical evidence available from which to create an accurate system.
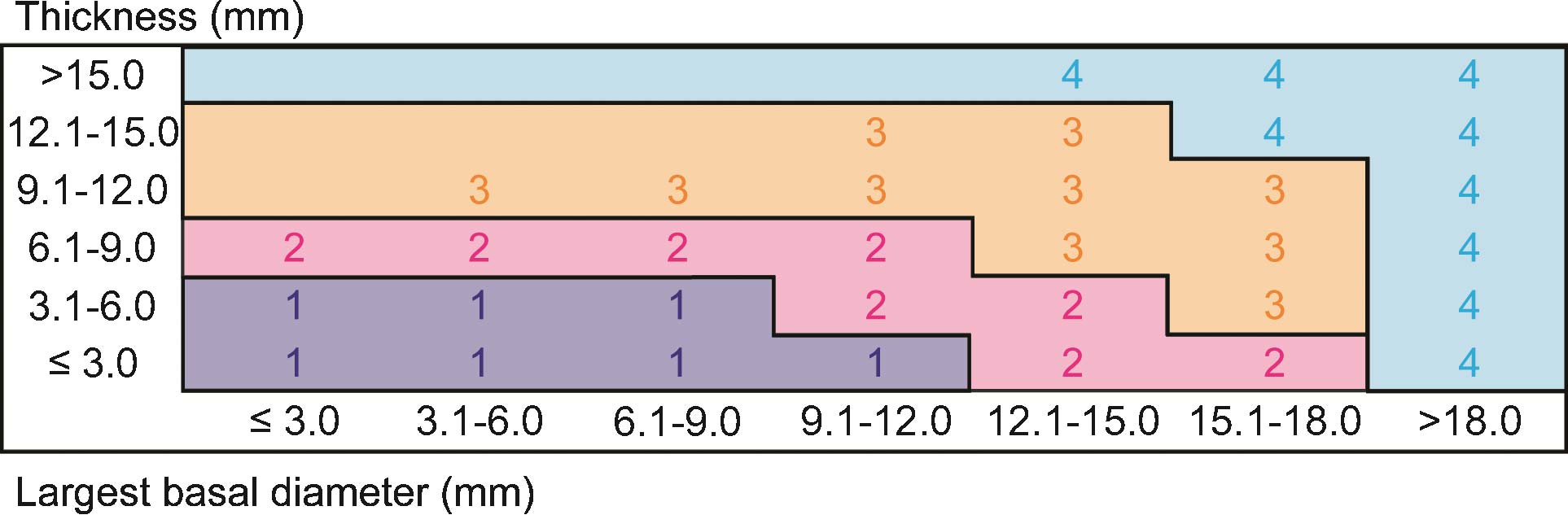
In contrast, the 7th Edition AJCC staging system for uveal melanoma was evidence based; it was empirically derived from a collaborative database of 7,369 patients.3 The previous T categories, based on cut points of tumor thickness and largest tumor basal diameter, were replaced by redefined T categories based on blocks representing 3x3-mm size fractions (Figure 86.1). In addition, this edition took into account involvement of the ciliary body and extrascleral tissues, the other predominant and independent clinical predictors of survival in uveal melanoma. For the first time, staging was defined so that categories of anatomic extent with mutually similar survival were assigned to the same stage. Empirical data were insufficient to propose a major revision of the AJCC staging system for iris melanomas.
During the past 5 years, the 7th Edition staging system for choroidal and ciliary body melanomas has been independently validated. Ten-year survival rates for the seven stages—I, IIA-B, IIIA-C, and IV—in the 5,403 patients in the original European dataset used to formulate the stages,3 and in a later international validation study of 3,217 patients,4 are clearly distinct between stages and consistent in these two large studies, especially with regard to 5-year survival (Table 86.1). Follow-up was shorter in the validation study, which likely explains the moderate differences in 10-year survival rates, especially in higher-stage categories, which contained fewer patients; however, the confidence intervals overlap. Several single-center studies and series of specific groups of patients, such as children and young adults, also have supported the validity of staging on the basis of anatomic extent.5-7
86.1 Stage-specific 5- and 10-year survival rates in the original and validation studies of staging for nonmetastatic primary choroidal and ciliary body melanomas3,4
| 5-Year survival rate | 10-Year survival rate | |||
|---|---|---|---|---|
| Stage | Original study (%) | Validation study (%) | Original study (%) | Validation study (%) |
| I | 96 (94-97) | 97 (95-98) | 88 (84-91) | 94 (91-96) |
| IIA | 89 (87-91) | 89 (86-91) | 80 (76-83) | 84 (80-88) |
| IIB | 81 (78-84) | 79 (75-83) | 67 (62-71) | 70 (62-76) |
| IIIA | 66 (62-70) | 67 (59-73) | 45 (39-51) | 60 (51-68) |
| IIIB | 45 (39-52) | 50 (33-65) | 27 (19-36) | 50 (33-65) |
| IIIC | 26 (13-40) | 25 (4-53) | N/A | N/A |
| N/A, not available because of a very small number of patients surviving; numbers in parentheses are 95% confidence intervals. | ||||
Although it would be ideal to implement cytogenetic, GEP, and molecular genetic prognosticators in the AJCC staging system for uveal melanoma, the aforementioned observations are too recent to allow that with confidence. Specifically, the average follow-up in patients with identified changes is less than 5 years, and the number of patients in many AJCC T size categories is still very small. Preliminary observations suggest that most patients with disomy 3 and probably those with the most favorable GEP class—1A—have a very small risk of metastasis, one that approaches the risk in Stage I or less.6 Conversely, patients in AJCC Stages IIA to IIIC have increasing proportions of uveal melanomas that show monosomy 3 and chromosome 8 changes, or represent GEP class 2. These patients likely are responsible for most of the variation seen in survival, by anatomic extent, in AJCC Stages IIA to IIIC.6 The question of how patients with a genetic signature of intermediate risk fit into this scheme is still open. A genetic amendment to the AJCC staging system eventually will reassign patients to stages according to a combination of genetic data and anatomic extent. As in the 7th and 8th Editions, such changes must be evidence based. Time will allow for the emergence of the most reliable and cost-effective genetic biomarkers to be included in the AJCC staging system for uveal melanoma. Lastly, it is important to note that AJCC anatomic staging continues to be invaluable when cytogenetic prognostication is unavailable or not offered to the patient.