Dementia is a progressive syndrome affecting around 5% of those aged over 65 years, rising to 20% in the over 80s. The disorder is characterised by cognitive decline, impaired memory and thinking and a gradual loss of skills needed to carry out activities of daily living (ADL). Changes in mood, personality and social behaviour are frequent.1
The various types of dementia are classified according to the different disease processes affecting the brain. The most common cause of dementia is Alzheimer's disease (AD), accounting for around 60% of all cases. Vascular dementia (VaD) and dementia with Lewy bodies (DLB) are responsible for most other cases. AD and VaD may coexist and are often difficult to separate clinically. Dementia is also encountered in about 30-70% of patients with Parkinson's disease1 (see Chapter 10).
The cholinergic hypothesis of AD is predicated on the observation that the cognitive deterioration associated with the disease results from progressive loss of cholinergic neurons and decreasing levels of acetylcholine (ACh) in the brain.2 However, it is no longer widely believed that cholinergic depletion alone is responsible for the symptoms of AD.3
Three inhibitors of AChE are currently licensed in the UK and elsewhere for the treatment of mild to moderate dementia in AD: donepezil, rivastigmine and galantamine. These three drugs are also recommended in severe AD. In addition, rivastigmine is licensed in some countries for the treatment of mild to moderate dementia associated with Parkinson's disease.
Both AChE and butyrylcholinesterase (BuChE) have been found to play an important role in the degradation of ACh.4 Cholinesterase inhibitors differ in pharmacological action: donepezil selectively inhibits AChE, rivastigmine affects both AChE and BuChE and galantamine selectively inhibits AChE and also has nicotinic receptor agonist properties.5 To date, these differences have not been shown to result in important differences in efficacy or tolerability (see Table 6.1 for a comparison of AChE inhibitors).
Table 6.1 Characteristics of Cognitive Enhancers.7891011121314
| Characteristic | Donepezil | Rivastigmine | Galantamine | Memantine |
|---|---|---|---|---|
| Primary mechanism | AChE-I (selective and reversible) | AChE-I (reversible, non-competitive inhibitor) | AChE-I (competitive and reversible) | Glutamate receptor antagonist |
| Other mechanism | None | BuChE-I | Nicotine receptor agonist | 5HT3 receptor antagonist |
Starting dose | 5mg daily | 1.5mg bd (oral) (or 4.6mg/24 hours patch) | 8mg XL daily (or 4mg bd solution) (immediate-release tablets largely discontinued) | 5mg daily |
| Usual treatment dose | 10mg daily | 3-6mg bd (oral) (or 9.5mg/24 hours patch) | 16-24mg XL daily (or 8-12mg bd solution) | 20mg daily (or 10mg bd) |
Recommended minimum interval between dose increases | 4 weeks (increase by 5mg daily) | 2 weeks for oral (increase by 1.5mg twice a day) 4 weeks for patch (increase to 9.5mg/24 hours) Consider increase to 13.3mg/24 hours after 6 months | 4 weeks (increase by 8mg XL daily or by 4mg bd for solution) | 1 week (increase by 5mg weekly) |
(*very common: ≥1/10 and common: ≥1/100) | Diarrhoea,* nausea,* headache,* common cold, anorexia, hallucinations, agitation, aggressive behaviour, abnormal dreams and nightmares, syncope, dizziness, insomnia, vomiting, rash, pruritis, muscle cramps, urinary incontinence, fatigue, pain, falls | Anorexia,* dizziness,* nausea,* vomiting,* diarrhoea,* decreased appetite, nightmares, agitation, confusion, anxiety, headache, somnolence, tremor, abdominal pain and dyspepsia, sweating, fatigue and asthenia, malaise, weight loss (frequency of adverse effects with the patch may differ) | Nausea,* vomiting,* decreased appetite, hallucination, depression, syncope, dizziness, tremor, headache, somnolence, lethargy, bradycardia, hypertension, abdominal pain and discomfort, diarrhoea, dyspepsia, muscle spasms, fatigue, asthenia, malaise, weight loss, fall, laceration | Drug hypersensitivity, somnolence, dizziness, balance disorders, hypertension, dyspnoea, constipation, elevated liver function test, headache |
Half-life (hours) | ~70 | ~1 (oral) 3.4 (patch) | 7-8 (oral solution) 8-10 (XL capsules) | 60-100 |
Metabolism | CYP3A4 CYP2D6 (minor) | Minimal involvement of CYP isoenzymes | CYP3A4 CYP2D6 | Primarily non-hepatic |
| Drug-drug interactions | Yes (see Table 6.2) | Interactions unlikely | Yes (see Table 6.2) | Yes (see Table 6.2) |
| Effect of food on absorption | None | Delays rate and extent of absorption | Delays rate but not extent of absorption | None |
AChE-I, acetylcholinesterase inhibitor; bd, twice a day; BuChE, butyrylcholinesterase.
Memantine is licensed in the UK and elsewhere for the treatment of moderate to severe dementia in AD. It is believed to exert its therapeutic effect by acting as a low to moderate affinity, non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist that binds preferentially to open NMDA receptor-operated calcium channels. This activity-dependent binding blocks NMDA-mediated ion flux and is thought to mitigate the effects of sustained and pathologically elevated levels of glutamate (and this excitotoxicity) that may lead to neuronal dysfunction (Table 6.1).6
Currently, no treatments exist that unequivocally reverse disease progression in dementia. Therapeutic interventions are therefore targeted at specific symptoms or at improving or slowing the decline in cognitive function. AChE inhibitors (AChE-Is) may provide some modest cognitive, functional and global benefits in mild to moderate AD.15
The three AChE-Is seem to have broadly similar clinical effects; estimates of the number needed to treat (NNT) (for an improvement of >4 points in the AD Assessment Scale - cognitive subscale [ADAS-cog]) range from 4 to 12.16
An analysis of memantine studies found estimated NNT ranged from 3 to 817 for improved cognitive function. A Cochrane review of memantine in dementia confirmed that there was a small clinical benefit of memantine in people with moderate to severe AD, which occurs irrespective of whether they are also taking a cholinesterase inhibitor, but no benefit in people with mild AD.17
A 2021 study18 investigated the ‘real world' effectiveness of cholinesterase inhibitors and memantine. The study found that, in general, the initial decline in Mini Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores occurred approximately 2 years before medication was eventually initiated. Medication stabilised cognitive performance for the ensuing 2-5 months. The effect was enhanced in more cognitively impaired cases and attenuated in those taking antipsychotics. Importantly, patients who were switched between agents at least once tended to continue to decline at their pre-medication rate (i.e. did not benefit from pharmacological interventions). Those who remained on the same agent tended to respond better and to stabilise in respect to cognitive changes for a period once the medication was prescribed. Of course, switching might be more common in non-responders, so the act of switching itself may not be detrimental to outcome. Overall, 68% of individuals experienced a period of cognitive stabilisation before continuing to decline at the pre-treatment rate. Other studies have found similar benefits alongside evidence that AChE-Is may reduce overall mortality.19
The benefits of treatment with AChE-Is are rapidly lost when drug administration is interrupted20 and may not be fully regained when drug treatment is reinitiated.21 Poor tolerability with one agent does not rule out good tolerability with another.22 The British Association for Psychopharmacology (BAP) guidelines for dementia confirm that previous comparative trials have failed to consistently demonstrate any significant differences in efficacy between the three AChE-Is, the main differences found being in frequency and type of adverse events. As a result, their recommendation remains valid that a significant proportion of patients (up to 50%) appear to both tolerate and benefit from switching between AChE-Is if they cannot tolerate one.23
Several cases of discontinuation syndrome upon stopping donepezil have been published24, 25 suggesting that a gradual withdrawal should be carried out where possible. However, a study comparing abrupt versus stepwise switching from donepezil to memantine found no clinically relevant differences in adverse effects despite patients in the abrupt group experiencing more frequent adverse effects than the stepwise discontinuation group (46% vs 32%, respectively).26 (For switching to rivastigmine patch see ‘Tolerability' later in this chapter.)
Following a systematic review of the literature,27 a practical approach to switching between AChE-Is has been proposed. In the case of intolerance, switching to another agent should be done only after complete resolution of side effects following discontinuation of the initial agent. In the case of lack of efficacy, switching can be done overnight, with a quicker titration scheme thereafter. Switching to another AChE-I is not recommended in individuals who show loss of benefit several years after initiation of therapy.
AChE-Is may also affect non-cognitive aspects of AD and other dementias. For more information about the management of these symptoms, see ‘Management of behavioural and psychological symptoms of dementia (BPSD)' later in this chapter.
For dosing information see Table 6.1 and up-to-date manufacturers' literature.
Rivastigmine transdermal patches (9.5mg/24 hours) have been shown to be as effective as the highest doses of capsules but with a superior tolerability profile in a 6-month double-blind placebo-controlled randomised controlled (RCT).28 This has been confirmed in a Chinese study.29 A nasal spray has also been developed.30
The US Food and Drug Administration (FDA) has approved a higher daily dose of donepezil sustained release (23mg) for moderate to severe AD. In the approval trial there was a small statistically significant improvement in cognition (a 2.2 improvement over the 10mg dose on the Severe Impairment Battery [SIB] scale) but no difference in global functioning (a 0.06 improvement on the Clinician's Interview-Based Impression of Change plus caregiver input [CIBIC-plus] scale). Furthermore, the higher dose was not superior on either of the prespecified secondary outcome measures and the rate of gastrointestinal adverse effects was over three times higher (21%) in the first month in the group receiving donepezil 23mg than in the 10mg group (5.9%).31
The memantine extended release (ER) 28mg once-daily capsule formulation was approved in the USA in 2010. Its efficacy was demonstrated in a large, multinational, phase III trial which showed that the addition of memantine ER to ongoing cholinesterase inhibitors improved key outcomes compared with cholinesterase inhibitor monotherapy, including measures of cognition and global status. The most common adverse events were headache, diarrhoea and dizziness.32 While the FDA chose to approve memantine ER based on efficacy data from this study, the European Medicines Agency decided against approval. It questioned the clinical relevance of the drug given the small differences on the co-primary endpoints and the non-significant differences on the functional measure. In addition, since no comparison studies were performed between memantine immediate release (IR) and memantine ER, the risk-benefit ratio could not be fully evaluated.33
These high doses of donepezil and memantine have not yet been approved in the UK and many other countries. In addition, most older people seen in practice with AD are likely to be frailer and have more comorbidities than patients in clinical trials and may therefore be less likely to tolerate the higher doses.
Guidelines and the UK's National Institute for Health and Care Excellence (NICE)1 recommend the use of a combination of AChE-I plus memantine rather than AChE-I alone in patients with moderate to severe AD. A network meta-analysis of 54 trials found that memantine plus donepezil showed superior outcomes for cognition, global assessment, daily activities and neuropsychiatric symptoms, but lower acceptability than monotherapy and placebo. A 2022 analysis observed broadly similar outcomes.34 Combination therapy may be more cost-effective because memantine slows the progression of AD.35 A Cochrane review has confirmed these recommendations for combined therapy.36 Studies have also shown that there are no pharmacokinetic or pharmacodynamic interactions between AChE-Is and memantine.37, 38
Drug tolerability may differ between AChE-Is, but, in the absence of sufficient direct comparisons, it is difficult to draw conclusions. Overall tolerability can be broadly evaluated by reference to the numbers withdrawing from clinical trials. Withdrawal rates in trials of donepezil39, 40 ranged from 4% to 16% (placebo 1-7%), 7% to 29% (placebo 7%) with rivastigmine41, 42 and 7% to 23% (placebo 7-9%) with galantamine.43, 44, 45 These figures relate to withdrawals specifically associated with adverse effects. The number needed to harm (NNH) has been reported to be 12.16 A study of the French pharmacovigilance database identified age and the use of antipsychotic drugs, antihypertensives and drugs targeting the alimentary tract and metabolism as factors associated with serious reactions to AChE-Is.46 It has also been suggested that donepezil and rivastigmine are active centrally (CNS events, extrapyramidal symptoms, sleep disturbances and cardiorespiratory events), in contrast to galantamine, which is more active peripherally (muscle cramps and weakness, cardiorespiratory events and urinary incontinence).47
Tolerability seems to be affected by the speed of titration and, perhaps less clearly, by dose. Most adverse effects occurred in trials during titration, and slower titration schedules are recommended in clinical use. This may mean that these drugs are equally well tolerated in practice.
Rivastigmine patches offer convenience and a superior tolerability profile to rivastigmine capsules.28, 29 Data from three trials found that rivastigmine patches were better tolerated than the capsules with fewer gastrointestinal adverse effects and fewer discontinuations due to these adverse effects.48 Data support recommendations for patients on high doses of rivastigmine capsules (>6mg/day) to switch directly to the 9.5mg/24 hours patch, while those on lower doses (≤6mg/day) should start on 4.6mg/hour patch for 4 weeks before increasing to the 9.5mg/hour patch. This latter switch is also recommended for patients switching from other oral cholinesterase inhibitors to the rivastigmine patch (with a 1-week washout period in patients sensitive to adverse effects or who have very low body weight or a history of bradycardia).49 It is possible to consider increasing the dose to 13.3mg/24 hours after 6 months on 9.5mg/24 hours if tolerated and cognitive or functional decline occurs on the lower dose. A 48-week RCT found the higher-strength patch (13.3mg) significantly reduced deterioration in instrumental activities of daily living (IADL) compared with the 9.5mg/24 hours patch and was well tolerated.50
Patients and caregivers should be instructed on important administration details for the rivastigmine patch:9
- The transdermal patch should not be applied to skin that is red, irritated or cut.
- Reapplication to the exact same skin location within 14 days should be avoided to minimise the potential risk of skin irritation.
- The previous day's patch must be removed before applying a new one every day.
- Only one patch should be worn at a time.
- The patch should not be cut into pieces.
The following cautions exist for the use of AChE-Is: asthma, chronic obstructive pulmonary disease (COPD), sick sinus syndrome, supraventricular conduction abnormalities, susceptibility to peptic ulcers, history of seizures, bladder (or gastrointestinal) outflow obstruction, cardiac disease, congestive heart failure, unstable angina, electrolyte disturbances; and for rivastigmine patches: risk of fatal overdose with patch administration errors.7
Memantine appears to be well tolerated52, 53 and the only conditions associated with warnings include severe hepatic impairment and epilepsy/seizures.54 (See BNF or equivalent for required dosage adjustments in renal impairment.) Isolated cases of international normalised ratio (INR) increases have been reported when memantine is co-administered with warfarin.
When adverse effects occur with AChE-Is they are largely predictable: excess cholinergic stimulation leads to nausea, vomiting, dizziness, insomnia and diarrhoea.55 Such effects are most likely to occur at the start of therapy or when the dose is increased. They are dose related and tend to be transient. Urinary incontinence has also been reported.56
A network meta-analysis57 compared efficacy and safety with these agents and found the following hierarchy in terms of tolerability:
- Withdrawals from studies due to adverse effects: donepezil > galantamine > rivastigmine patch > rivastigmine (meaning donepezil is best tolerated and so on).
- Nausea: rivastigmine patch > donepezil > galantamine > rivastigmine.
- Vomiting: donepezil > rivastigmine patch > galantamine > rivastigmine.
- Diarrhoea: galantamine > rivastigmine > rivastigmine patch > donepezil.
- Dizziness: rivastigmine patch > galantamine > donepezil > rivastigmine.
An analysis of 16 years of individual case safety reports from VigiBase found that the most common adverse effects reported with AChE-Is were neuropsychiatric symptoms (31.4%), gastrointestinal disorders (15.9%) and general disorders and administration site conditions (11.9%). Cardiovascular adverse drug reactions (ADRs) accounted for 11.7% of ADRs.58
In view of their pharmacological action, AChE-Is can be expected to have vagotonic effects on the heart rate (i.e. bradycardia). The potential for this action may be of particular importance in patients with sick sinus syndrome or other supraventricular cardiac conduction disturbances, such as sinoatrial or atrioventricular block.7, 8, 9, 10, 11, 12
Concerns over the potential cardiac adverse effects associated with AChE-Is were raised following findings from controlled trials of galantamine in mild cognitive impairment (MCI) in which increased mortality was associated with galantamine compared with placebo (1.5% vs 0.5%).59 Although no specific cause of death was dominant, half the deaths reported were due to cardiovascular disorders. As a result, the FDA issued a warning restricting galantamine in patients with MCI. The relevance to AD remains unclear.60
The most prominent cardiovascular adverse effects of AChE-Is are bradycardia and syncope, which can result in serious outcomes such as falls, fractures and other trauma as well as necessitate pacemaker placement. If these adverse effects are experienced, patients should undergo a thorough history/evaluation, including a medication review, rhythm monitoring, consideration of neurological symptoms, lowering the doses of other medications that might contribute to bradycardia, stopping or reducing the AChE-I dose or even pacemaker placement. Many of these factors should be considered before the initiation of these medications and periodically thereafter to optimise patient care and mitigate possible adverse events61, 62 (Figure ). There are also a few reports that they may occasionally be associated with QT prolongation and torsades de pointes.63
Reproduced with permission from Rowland et al. 2007, © 2007 by The Royal College of Psychiatrists.
Figure. 6.2.1 Suggested Guidelines for Managing Cardiovascular Risk Prior to and During Treatment with Acetylcholinesterase Inhibitors (AChE-Is) in Alzheimer's Disease. Bpm, Beats Per Minute. Reproduced with Permission from Rowland Et Al. 2007, © 2007 by The Royal College of Psychiatrists.
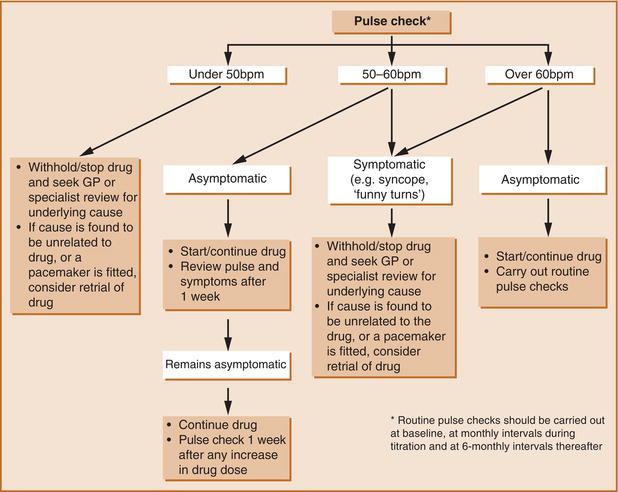
It seems that patients with DLB are more susceptible to the bradyarrhythmic adverse effects of these drugs owing to the autonomic insufficiency associated with the disease.64
The manufacturers of all three agents advise that the drugs should be used with caution in patients with cardiovascular disease or in those taking concurrent medicines that reduce heart rate (e.g. digoxin or β blockers). Although a pre-treatment mandatory electrocardiogram (ECG) has been suggested,60 a review of published evidence showed that the incidence of cardiovascular side effects is low and that serious adverse effects are rare. In addition, the value of pre-treatment screening and routine ECGs is questionable and is not currently recommended by NICE. However, in patients with a history of cardiovascular disease or who are prescribed concomitant negative chronotropic drugs with AChE-Is, an ECG is advised.61
Although little is known about the cardiovascular effects of memantine, there have been reports of bradycardia and reduced cardiovascular survival associated with its use.65
An analysis of pooled prospective data for memantine revealed that the most frequently reported adverse effects in placebo-controlled trials included agitation (7.5% memantine vs 12% placebo), falls (6.8% vs 7.1%), dizziness (6.3% vs 5.7%), accidental injury (6.0% vs 7.2%), influenza-like symptoms (6.0% vs 5.8%), headache (5.2% vs 3.7%) and diarrhoea (5.0% vs 5.6%).66 Given the higher or similar rates seen with placebo, few conclusions can be drawn.
The French pharmacovigilance database compared adverse effects reported with donepezil with memantine. The most frequent ADRs with donepezil alone and memantine alone were respectively bradycardia (10% vs 7%), weakness (5% vs 6%) and convulsions (4% vs 3%). Although it is well known that donepezil is often associated with bradycardia and memantine associated with seizures, this analysis suggested that memantine can also induce bradycardia and donepezil seizures, thus highlighting the care required when treating patients with dementia who have a history of bradycardia or epilepsy.67
Potential for interaction may also differentiate currently available cholinesterase inhibitors. Donepezil68 and galantamine69 are metabolised by cytochromes 2D6 and 3A4 so drug levels may be altered by other drugs affecting the function of these enzymes. Cholinesterase inhibitors themselves may also interfere with the metabolism of other drugs, although this is perhaps a theoretical consideration. Rivastigmine has almost no potential for interaction since it is metabolised at the site of action and does not affect hepatic cytochromes. A prospective pharmacodynamic analysis of potential drug interactions between rivastigmine and other medications (22 different therapeutic classes) commonly prescribed in the elderly population compared adverse effects odds ratios between rivastigmine and placebo. Rivastigmine was not associated with any significant pattern of increase in adverse effects that would indicate a drug interaction compared with placebo.70 Rivastigmine thus appears to be least likely to cause problematic drug interactions, a factor that may be important in an elderly population subject to polypharmacy (Table 6.2).
Table 6.2 Drug-Drug Interactions.891011127475
| Drug | Metabolism | Plasma levels increased by | Plasma levels decreased by | Pharmacodynamic interactions |
|---|---|---|---|---|
Donepezil (Aricept®) | Substrate at 3A4 and 2D6 | Ketoconazole Itraconazole Erythromycin Quinidine Fluoxetine Paroxetine | Rifampicin Phenytoin Carbamazepine Alcohol | Antagonistic with anticholinergic drugs and competitive neuromuscular blockers (e.g. tubocurarine). Potential for synergistic activity with cholinomimetics such as depolarising neuromuscular blocking agents (e.g. succinylcholine), cholinergic agonists and peripherally acting cholinesterase inhibitors (e.g. neostigmine). Beta blockers, amiodarone or calcium channel blockers may have additive effects on cardiac conduction. Caution with concomitant use of drugs known to induce QT prolongation and/or torsades de pointes. Movement disorders and neuroleptic malignant syndrome have occurred with concomitant use of antipsychotics and cholinesterase inhibitors. Concurrent use with seizure lowering agents may result in reduced seizure threshold. |
Rivastigmine (Exelon®) | Non-hepatic metabolism | Metabolic interactions appear unlikely Smoking tobacco increases the clearance of rivastigmine | Antagonistic effects with anticholinergic and competitive neuromuscular blockers (e.g. tubocurarine). Potential for synergistic activity with cholinomimetics such as depolarising neuromuscular blocking agents (e.g. succinylcholine), cholinergic agonists (e.g. bethanecol) or peripherally acting cholinesterase inhibitors (e.g. neostigmine). Synergistic effects on cardiac conduction with β blockers, amiodarone and calcium channel blockers. Caution with concomitant use of drugs known to induce QT prolongation and/or torsades de pointes. Movement disorders and neuroleptic malignant syndrome have occurred with concomitant use of antipsychotics and cholinesterase inhibitors. Concurrent use with metoclopramide may result in increased risk of EPSEs. | |
Galantamine (Reminyl®) | Substrate at 3A4 and 2D6 | Ketoconazole Erythromycin Ritonavir Quinidine Paroxetine Fluoxetine Fluvoxamine Amitriptyline | None known | Antagonistic effects with anticholinergic and competitive neuromuscular blockers (e.g. tubocurarine). Potential for synergistic activity with cholinomimetics such as depolarising neuromuscular blocking agents (e.g. succinylcholine), cholinergic agonists and peripherally acting cholinesterase inhibitors (e.g. neostigmine). Possible interaction with agents that significantly reduce heart rate such as digoxin, β blockers, certain calcium channel blockers and amiodarone. Caution with concomitant use of drugs known to induce QT prolongation and/or torsades de pointes (manufacturer recommends ECG in such cases). Movement disorders and neuroleptic malignant syndrome have occurred with concomitant use of antipsychotics and cholinesterase inhibitors. |
Memantine (Exiba®) | Primarily non-hepatic metabolism Renally eliminated | Cimetidine Ranitidine Procainamide Quinidine Quinine Nicotine Trimethoprim Isolated cases of INR increases reported with concomitant warfarin (close monitoring of prothrombin time or INR advisable) Drugs that alkalinise urine (pH ~8) may reduce renal elimination of memantine, e.g. carbonic anhydrase inhibitors, sodium bicarbonate | None known (Possibility of reduced serum level of hydrochlorothiazide when co-administered with memantine) | Effects of L-dopa, dopaminergic agonists, selegiline and anticholinergics may be enhanced. Effects of barbiturates and antipsychotics may be reduced. Avoid concomitant use with amantadine, ketamine and dextromethorphan - increased risk of CNS toxicity. One published case report on possible risk for phenytoin and memantine combination. Dosage adjustment may be necessary for antispasmodic agents, dantrolene or baclofen when administered with memantine. A single case report of myoclonus and confusion when co-administered with co-trimoxazole or trimethoprim |
NB This list is not exhaustive. Take caution with other drugs that are also inhibitors or enhancers of CYP3A4 and CYP2D6 enzymes.
EPSEs, extrapyramidal side effects; INR, international normalised ratio.
Analysis of the French pharmacovigilance database found that the majority of reported drug interactions concerning AChE-Is were pharmacodynamic in nature and most frequently involved the combination of AChE-I and bradycardic drugs (β blockers, digoxin, amiodarone, calcium channel antagonists). Almost a third of these interactions resulted in cardiovascular ADRs such as bradycardia, atrioventricular block and arterial hypotension. The second most frequent drug interaction reported was the combination of AChE-I with anticholinergic drugs leading to pharmacological antagonism.71
The pharmacodynamics, pharmacokinetic and pharmacogenetic aspects of drugs used in dementia have been summarised in two comprehensive reviews.72, 73
A large multicentre study76 of community-dwelling patients with moderate or severe AD investigated the long-term effects of donepezil over 12 months compared with stopping donepezil after 3 months, switching to memantine or combining donepezil with memantine. Continued treatment with donepezil was associated with continued cognitive benefits, and patients with an MMSE score as low as 3 also benefitted from treatment. This suggests that patients should continue treatment with AChE-Is for as long as possible and there should not be a cut-off MMSE score where treatment is stopped automatically. Moreover, secondary and post-hoc analyses of this study found that withdrawal of donepezil in patients with moderate to severe AD increased the risk of nursing home placement during 12 months of treatment but made no difference during the following 3 years of follow-up. This highlights the point that decisions to stop or continue treatment should be informed by potential risks of withdrawal, even if the perceived benefits of continued treatment are not clear.77 A 2021 Cochrane review came to similar conclusions.36
The consensus opinion is that if the drug is well tolerated and the patient's physical health is stable, then it is probably best to continue the drug. The risks of discontinuation of dementia medication should be balanced against the adverse effects.78
In addition to this, a meta-analysis evaluating the efficacy of the three AChE-Is and memantine in relation to the severity of AD found that the efficacy of all drugs except memantine was independent of dementia severity in all domains. The effect of memantine on functional impairment was actually better in patients with more severe AD. This suggests that the severity of a patient's illness should not preclude treatment with these drugs.79
Guidance for discontinuation of dementia medication in clinical practice is summarised here.80
- When the patient/caregiver decides to stop (after being advised on the risks and benefits of stopping treatment).
- When the patient refuses to take the medication (but see ‘Covert administration of medicines within food and drink' later in this chapter).
- When there are problems with patient compliance which cannot be reasonably resolved.
- When the patient's cognitive, functional or behavioural decline is worsened by treatment.
- When there are intolerable adverse effects.
- When comorbidities make treatment risky or futile (e.g. terminal illness).
- Where there is no clinically meaningful benefit to continuing therapy (clinical judgement should be used here rather than ceasing treatment when a patient reaches a certain score on a cognitive outcome or when they are institutionalised).
- When dementia has progressed to a severely impaired stage (Global Deterioration Scale stage 7: development of swallowing difficulties).
When a decision is made to stop therapy (for reasons other than lack of tolerability), tapering of the dose and monitoring the patient for evidence of significant decline during the next 1-3 months are advised. If such decline occurs, reinstatement of therapy should be considered.
NICE guidance on dementia81 was last updated in June 2018 (Box 6.2).
|
Summary of NICE Guidance for the Treatment of Non-AD Dementia81, 82
|
Medicines that May Cause Cognitive Impairment1
|
A 2009 Cochrane review84 concluded that Ginkgo biloba appears to be safe in use with no excess adverse effects compared with placebo, but the evidence that it has predictable and clinically significant benefit for people with dementia or cognitive impairment is inconsistent and unreliable. In contrast, a 2015 systematic review and meta-analysis85 found that Ginkgo biloba 240mg/day was able to stabilise or slow decline in cognition, function, behaviour and global change at 22-26 weeks in patients with cognitive impairment and dementia, especially for patients with neuropsychiatric symptoms. A 2022 umbrella review confirmed the efficacy of Ginkgo.86 Several reports have noted that Ginkgo may increase the risk of bleeding.87
The findings of a systematic review88 suggest that supplementation of B complex vitamins, especially folic acid, may have a positive effect on delaying and preventing the risk of cognitive decline. Ascorbic acid and a high dose of vitamin E, when given separately, also showed positive effects on cognitive performance, but there is not sufficient evidence to support their use. The results of vitamin D supplementation trials are not conclusive in assessing the potential benefits that vitamin D might have on cognition.
A Cochrane review of omega-3 fatty acids for the treatment of dementia (632 people with mild to moderate AD) found that taking omega-3 polyunsaturated fatty acid supplements for 6 months had no effect on cognition (learning and understanding), everyday functioning, quality of life or mental health. The trials did not report side effects very well, but none of the studies described significant harmful effects on health.89
A systematic review and meta-analysis including four RCTs involving 259 participants suggested that the effects of ginseng on AD remain unproven.90
Natural hirudin, isolated from the salivary gland of the medicinal leech, is a direct thrombin inhibitor and has been used for many years in China. A small 20-week open-label RCT of 84 patients receiving donepezil or donepezil plus hirudin (3g/day) found that patients on the combination showed significant decrease in ADAS-cog scores and significant increase in ADL scores compared with donepezil alone. However, haemorrhage and hypersensitivity reactions were more common in the combination group than in the donepezil group (11.9% and 7.1% vs 2.4% and 2.4%, respectively).78 The potential haemorrhagic effects of hirudin need further exploration before it can be considered for clinical use.
Huperzine A, an alkaloid isolated from the Chinese herb Huperzia serrata, is a potent, highly selective, reversible AChE-I used for treating AD since 1994 in China and available as a nutraceutical in the USA. Despite its promising effects on cognition and ADLs, there is insufficient evidence to support its use in dementia91 or MCI91, 92 due to the high heterogeneity of reviews and low quality of primary studies. High-quality, large, multicentre RCTs with long-term follow-up in different settings are warranted but no studies have been published since 2020. A Cochrane review of huperzine A in VaD found no convincing evidence for its value in VaD.93
There is increasing evidence to suggest possible efficacy of Crocus sativus (saffron) in the management of AD. A systematic review and meta-analysis of RCTs revealed that saffron significantly improves cognitive function measured by ADAS-cog and the Clinical Dementia Rating Scale - Sums of Boxes (CDR-SB) compared with placebo groups. In addition, there was no difference between saffron and conventional medicines (donepezil, memantine). No serious adverse events were reported in the included studies. Saffron may be beneficial in improving cognitive function in patients with MCI and AD, however no evidence was found to support its effects on other types of dementia. More high-quality randomised placebo-controlled trials are needed to further confirm the efficacy and safety of saffron for MCI and dementia.94
Cerebrolysin is a parenterally administered, porcine brain-derived peptide preparation that has pharmacodynamic properties similar to those of endogenous neurotrophic factors. A meta-analysis included six RCTs comparing cerebrolysin 30mg/day with placebo in mild to moderate AD. Cerebrolysin was more effective than placebo at 4 weeks regarding cognitive function and at 4 weeks and 6 months regarding global clinical change and 'global benefit'. Its safety was comparable with placebo. In addition, a large 28-week RCT comparing cerebrolysin, donepezil or combination therapy showed (i) higher improvements in global outcome for cerebrolysin and the combination therapy than for donepezil alone at study endpoint; (ii) a lack of significant group differences in cognitive, functional and behavioural domains at the endpoint; and (iii) best scores of cognitive improvement in the combination therapy group at all study visits.95 This therapeutic option requires further investigation in large trials.
A Cochrane review assessing cerebrolysin in VaD found that intravenous courses improved cognition and general function in people living with VaD, with no suggestion of adverse effects. However, these data are not definitive. The analyses were limited by heterogeneity, and studies had high risk of bias. If there are benefits, the effects may be too small to be clinically meaningful. Cerebrolysin continues to be used and promoted as a treatment for VaD, but the supporting evidence base is weak. The most commonly reported non-serious adverse events were headache, aesthenia, dizziness, hypertension and hypotension.96
For information on statins see ‘Safer prescribing for physical conditions in dementia' later in this chapter.
A longitudinal prospective study examined the relationship between chocolate consumption and cognitive decline in an elderly cognitively healthy population. A total of 531 participants aged ≥65 years with normal MMSE scores were followed for a median of 48 months. Dietary habits were evaluated at baseline and the MMSE was used to assess global cognitive function at baseline and at follow-up. After adjustment for confounders, chocolate intake was associated with a 41% lower risk of cognitive decline. This protective effect was observed only among subjects with an average daily consumption of caffeine lower than 75mg.97
Souvenaid is a medical food for the dietary management of early AD. A Cochrane review98 concluded that it probably does not reduce the risk of progression to dementia, there is no convincing evidence that it affects other outcomes important to people with AD (in the prodromal stage or mild to moderate stages) and its effects in more severe AD remain unclear.
Idalopirdine is a 5HT6 receptor antagonist. The 5HT6 receptor is expressed in areas of the CNS involved with memory and there is evidence suggesting that blocking of these receptors induces acetylcholine release and could restore ACh levels in a deteriorated cholinergic system.99 A systematic review and meta-analysis analysed four RCTs with 2803 patients with AD. Idalopirdine was not shown to be effective for AD patients and is associated with a risk of elevated liver enzymes and vomiting. Although idalopirdine might be more effective at high doses and in moderate AD subgroups, the effect size is small.100
A large number of RCTs of anti-inflammatory drugs in AD have failed to reach primary outcomes. Large-scale studies of non-steroidal anti-inflammatory drugs (NSAIDs) including indomethacin, naproxen and rofecoxib in AD have been unsuccessful. RCTs with a range of other anti-inflammatory drugs including prednisolone, hydroxychloroquine, simvastatin, atorvastatin, aspirin and rosiglitazone have also shown no clinically significant changes in primary cognitive outcomes in patients with AD.23 A 2020 Cochrane review evaluated aspirin and other NSAIDs for the prevention of dementia and found no evidence to support the use of low-dose aspirin or other NSAIDs of any class (celecoxib, rofecoxib, naproxen) for the prevention of dementia. There was, however, evidence of harm including higher rates of death and major bleeding compared with placebo with aspirin, and in one of the studies more people developed dementia in the NSAID group. More stomach bleeding and other stomach problems, such as pain, nausea and gastritis, were also reported with NSAIDs.101
Two existing compounds, trazodone and dibenzoylmethane, were found to be markedly neuroprotective in mouse models of neurodegeneration, using clinically relevant doses over a prolonged period of time, without systemic toxicity. Trazodone, a serotonin antagonist and reuptake antidepressant with additional anxiolytic and hypnotic effects, was associated with delayed cognitive decline in a small retrospective study examining its long-term use. Trazodone non-users had a 2.6-fold faster decline in MMSE (primary outcome) assessment than trazodone users.102 However, a study of UK population-based electronic health records found no association between trazodone use and a reduced risk of dementia compared with other antidepressants. These results suggest that the clinical use of trazodone is not associated with a reduced risk of dementia.103 Similarly, three identical naturalistic cohort studies using UK clinical registers found no evidence of cognitive benefit from trazodone compared with other antidepressants in people with dementia.103 Despite pre-clinical evidence, trazodone should not be prescribed for cognition in dementia.104 There are no observational data suggesting trazodone reduces risk of dementia but some data that suggest important adverse outcomes in older people.105 Dibenzoylmethane (DBM) is a minor constituent of liquorice that has been found to have antineoplastic effects, with efficacy against prostate and mammary tumours. In prion-diseased mice, both trazodone and DBM treatment restored memory deficits, abrogated the development of neurological signs, prevented neurodegeneration and significantly prolonged survival. In tauopathy-frontotemporal dementia mice, both drugs were neuroprotective, rescued memory deficits and reduced hippocampal atrophy. Further, trazodone reduced p-tau burden.106
KarXT (xanomeline plus trospium (Cobenfy)) is an investigational treatment that has shown early promise in the treatment of positive and negative symptoms of schizophrenia. Unlike all currently approved treatments for schizophrenia, KarXT does not directly bind to dopamine receptors; instead, the therapeutic effects of KarXT appear to be mediated through direct agonism of muscarinic acetylcholine receptors. To mitigate the cholinomimetic effects of xanomeline (e.g. vomiting), trospium is combined with xanomeline. Findings suggest that KarXT may have a separable and meaningful impact on cognition, particularly among patients with cognitive impairment.107
Quercetin is a flavonoid widely distributed among plants and found commonly in our daily diet (fruits and vegetables). It has beneficial properties against general mechanisms of AD aetiology; it protects neuronal cells by attenuating oxidative stress and neuroinflammation. Quercetin inhibits β-amyloid (Aβ) aggregation and tau phosphorylation and restores acetylcholine levels through the inhibition of hydrolysis of acetylcholine by AChE enzyme. Although showing neuroprotective efficacy in several in vitro and animal models, in vivo studies have reported that it is extensively metabolised upon absorption from the gut, affecting its bioavailability, and has low blood-brain barrier penetrability, thus limiting its efficacy in combating neurodegenerative disorders. Therefore, future clinical trials must improve its bioavailability, developing related molecules with greater gut and brain penetrability, which will likely improve clinical efficacy.108
Amyloid plaques are composed of β-amyloid (Aβ) in the extracellular space. Aβ is derived from the amyloid precursor protein (APP), a transmembrane protein. β secretase and &ggr secretase cleave the APP and generate pathological Aβ, and accumulation of Aβ results in neurotoxicity. Reducing the accumulation of Aβ has become a therapeutic purpose of AD. Antiamyloid therapy consists of three strategies: secretase inhibitors, Aβ aggregation inhibitors and Aβ immunotherapy.109
Aducanumab is an antibody that works by targeting Aβ and preferentially binds to the aggregated Aβ. Through this interaction, aducanumab could reduce the build-up of Aβ and therefore the number of amyloid plaques present in the brain, thus potentially slowing neurodegeneration and disease progression. Although in early 2019 the manufacturers (Biogen) announced that aducanumab failed futility analyses in two identically designed phase III AD trials and discontinued its development, later in the year they made the announcement that they were applying for US FDA marketing approval. They explained they had reanalysed data from the trials to include patients who had continued in the studies after the cut-off date for the futility analyses and stated that one trial showed significant findings and a subset from the second trial supports these positive findings.110 One concern with aducanumab was the frequency of adverse effects, particularly amyloid-related imaging abnormalities (ARIAs). In June 2021, the FDA made the decision to grant conditional accelerated approval for aducanumab to treat AD patients. Aducanumab was not approved in Europe.
The phase III trial of lecanemab (Clarity-AD trial) was more encouraging. Lecanemab lowers brain Aβ plaque burden through binding to soluble Aβ protofibrils as well as (to a variable extent) other forms of Aβ. The study included 1795 participants with MCI or early AD plus evidence of amyloid on a positron emission tomography (PET) scan or by cerebrospinal fluid testing. They were randomly assigned to receive 10mg/kg body weight of lecanemab via intravenous infusion every 2 weeks or matched placebo. After 18 months, lecanemab reduced cognitive decline, as measured by CDR-SB, which quantifies symptom severity across a range of cognitive and functional domains, by 27% compared with placebo; an absolute difference of 0.45 points (change from baseline 1.21 for lecanemab vs 1.66 with placebo, p <0.001). All key secondary endpoints were also met. The incidence of ARIAs, which manifest as oedema or microhaemorrhages, was 21% of the lecanemab group. Most cases were asymptomatic and detected incidentally. However, reports of deaths in the open-label extension phase of the study (possibly linked to co-administration of the thrombolytic drug alteplase) have heightened concerns about lecanemab's safety in patients taking thrombolytic drugs.111, 112 Lecanemab has been approved by the FDA and was undergoing a full evaluation by the European Medicines Agency at the time of writing.113
Donanemab is another high-potency antiamyloid drug infused intravenously every 4 weeks. In 2022, results were announced for the phase III trial (TRAILBLAZER-ALZ 2 trial) which included 1736 participants with early symptomatic AD (MCI/mild dementia) with amyloid and low/medium or high tau pathology based on PET imaging. Compared with placebo, donanemab treatment over 18 months resulted in slowing of cognitive and functional decline by approximately 35% in the primary target population studied. In addition, 52% of treated participants converted to amyloid PET-negative status by 12 months. ARIA-E (with oedema) and ARIA-H (with microhaemorrhage/haemosiderosis) occurred in 24.0% and 31.4% of treated individuals, respectively.114, 115 Donanemab is, at the time of writing, undergoing a full evaluation by the FDA and NICE.
The development of three monoclonal antibodies, gosuranemab, tilavonemab and zagotenemab, was terminated due to negative results. A phase II study of semorinemab, an anti-tau monoclonal antibody, was negative. While semorinemab had a significant effect on cognition measured by the ADAS-Cog11, this effect did not extend to improved functional or global outcomes.116 Further exploration is required. Clinical trials of anti-tau vaccines are underway.
In addition to the above, results of recent trials of solanezumab, crenezumab and gantenerumab were all negative.
Vascular dementia comprises 10-50% of dementia cases and is the second most common type of dementia after AD. It is caused by ischaemic damage to the brain and is associated with cognitive impairment and behavioural disturbances. The management options are currently very limited and focus on controlling the underlying risk factors for cerebrovascular disease.117
Note that it is impossible to diagnose with certainty vascular or Alzheimer's dementia and much dementia has mixed causation. This might explain why certain AChE-Is do not always provide consistent results in probable VaD and the data indicating efficacy in cognitive outcomes were derived from older patients, who were therefore likely to have concomitant AD pathology.118
None of the currently available drugs is formally licensed in the UK for VaD. The management of VaD has been summarised.119, 120 Unlike the situation with stroke, there is no conclusive evidence that treatment of hyperlipidaemia with statins or treatment of blood clotting abnormalities with acetylsalicylic acid has an effect on VaD incidence or disease progression.121 Similarly, a Cochrane review found that there were no studies supporting the role of statins in the treatment of VaD.122 A Cochrane review of cholinesterase inhibitors for VaD and other vascular cognitive impairments found moderate- to high-certainty evidence that donepezil 5mg, donepezil 10mg and galantamine 16-24mg have a slight beneficial effect on cognition in people with vascular cognitive impairment, although the size of the change is unlikely to be clinically important. Donepezil 10mg and galantamine 16-24mg are probably associated with more adverse events than placebo. The evidence for rivastigmine was less certain. Data suggest that donepezil 10mg has the greatest effect on cognition, but at the cost of adverse effects. The effect is modest, but in the absence of any other treatments, these agents may be considered in people living with vascular cognitive impairments. Further research into rivastigmine is needed, including the use of transdermal patches.123
A meta-analysis of RCTs found that cholinesterase inhibitors and memantine produce small benefits in cognition of uncertain clinical significance and concluded that data were insufficient to support widespread use of these agents in VaD; the effect is lower than that seen in AD, although no direct comparison has been made.117 A systematic review and Bayesian network meta-analysis comparing the efficacy and safety of cognitive enhancers for treating vascular cognitive impairment found significant efficacy for donepezil, galantamine and memantine on cognition. Memantine was found to provide significant efficacy in global status. They were all safe and well tolerated.124
DLB may account for 15-25% of cases of dementia. Characteristic symptoms are dementia with fluctuation of cognitive ability, early and persistent visual hallucinations and spontaneous motor features of parkinsonism. Falls, syncope, transient disturbances of consciousness, neuroleptic sensitivity and hallucinations in other modalities are also common.125
There are significant complexities in managing an individual with DLB. Presentation varies between patients and can vary over time within an individual. Treatments can address one symptom but worsen another, which makes disease management difficult. Symptoms are often managed in isolation and by different specialists, which makes high-quality care difficult to accomplish. Clinical trials and meta-analyses now provide an evidence base for the treatment of cognitive, neuropsychiatric and motor symptoms in patients with DLB.126 In summary, robust evidence exists for the efficacy of rivastigmine and donepezil in the treatment of cognitive symptoms in patients with DLB, but high-quality RCTs of galantamine are needed. Memantine could have some benefits, but further studies with larger numbers of patients are also needed to determine whether there is an improvement and, if so, which specific symptoms are improved. Whether memantine should be used as a monotherapy or whether it should be combined with cholinesterase inhibitors is also unclear.126, 127
For a helpful guide on the management of specific symptoms in DLB see the management of DLB summary sheets.128
The 2018 update of the NICE guidelines1 recommends the use of AChE-Is and memantine (if AChE-Is are not tolerated) in DLB and Parkinson's disease dementia (see Box 6.2).
Mild cognitive impairment is hypothesised to represent a pre-clinical stage of dementia but forms a heterogeneous group with variable prognosis. A Cochrane review assessing the safety and efficacy of AChE-Is in MCI found there was very little evidence that they affect progression to dementia or cognitive test scores. This weak evidence was countered by the increased risk of adverse effects, particularly gastrointestinal effects, meaning that AChE-Is could not be recommended in MCI.129 A systematic review130 found that there was no replicated evidence that any intervention was effective for MCI including AChE-Is and the NSAID rofecoxib. A further systematic review and meta-analysis found that although AChE-Is have a slight efficacy in the treatment of MCI, there are many safety issues, therefore they are difficult to recommend for MCI.131 Experts from several different countries have reviewed the available evidence for the pharmacological and non-pharmacological treatment for MCI.132, 133
A systemic review of RCTs for frontotemporal dementias showed that certain drugs may be effective in reducing behavioural symptoms (e.g. SSRIs, trazodone) but none of these had an effect on cognition.134 Due to new techniques in neuroimaging, genetics and biomarker analysis, much has been discovered about the phenomena underlying frontotemporal lobar degeneration. This has allowed the design of new molecule-based therapies that are still in the early stages of research but may show promise.135
A Cochrane review assessed the efficacy and safety of AChE-Is for rare dementias associated with neurological conditions. The sample sizes of most trials were very small and efficacy on cognitive function was found to be unclear, although AChE-Is were associated with more gastrointestinal adverse effects than placebo.136
AChE-Is and memantine are effective in AD of a broad range of severity. Other drugs including statins, anti-inflammatory drugs, vitamin E, nutritional supplements and Gingko cannot be recommended either for the treatment or prevention of AD. Neither AChE-Is nor memantine are effective in MCI. AChE-Is are not effective in frontotemporal dementia and may cause agitation. AChE-Is may be used for people with Lewy body dementia (both Parkinson's disease dementia and DLB), and memantine may be helpful. No drugs are clearly effective in VaD, though AChE-Is are beneficial in mixed dementia. Early evidence suggests multifactorial interventions may have the potential to prevent or delay the onset of dementia. Many novel pharmacological approaches involving strategies to reduce amyloid and/or tau deposition in those with or at high risk of AD are in progress. Although results of pivotal studies in early (prodromal/mild) AD are awaited, results to date in more established (mild to moderate) AD have been equivocal and no disease-modifying agents are either licensed or can be currently recommended for clinical use.
Table 6.3 summarises the clinical practice guidelines from BAP.23
Table 6.3 Summary of British Association for Psychopharmacology Recommendations.
| First choice | Second choice | |
|---|---|---|
| Alzheimer's disease | AChE-Is | Memantine |
| Vascular dementia | None (some benefit with donepezil 10mg - but risk of adverse effects) | None |
| Mixed dementia | AChE-Is | Memantine |
| Dementia with Lewy bodies | AChE-Is | Memantine |
| Mild cognitive impairment | None | None |
| Dementia with Parkinson's disease | AChE-Is | Memantine |
| Frontotemporal dementia | None | None |
AChE-Is, acetylcholinesterase inhibitors.
NB The Anticholinergic Effect on Cognition (AEC) scale can be accessed at www.medichec.com.
AChE-I, acetylcholinesterase inhibitors; AD, Alzheimer's disease; DLB, dementia with Lewy bodies; VaD, vascular dementia.