AUTHORS: Ned Premyodhin, MD and Pranav M. Patel, MD, FACC, FAHA, FSCAI
Kawasaki disease (KD) is an acute, febrile illness of unknown etiology that predominantly affects children <5 yr. It is the most common cause of acquired heart disease in children in developed countries. The pathology demonstrates a vasculitis of small- and medium-size blood vessels, with a predilection for the coronary arteries, which can result in coronary artery aneurysms and lesions. It is usually a self-limiting condition lasting an average of 12 days if not treated. Box E1 summarizes diagnostic criteria for classic or typical KD.1
BOX E1 Diagnostic Criteria for Classic or Typical Kawasaki Disease
From Cherry JD et al: Feigin and Cherry’s pediatric infectious diseases, ed 8, Philadelphia, 2019, Elsevier.
Mucocutaneous lymph node syndrome
| ||||||||
- Cause is currently unknown.
- KD is the leading cause of acquired heart disease in children in developed countries, including the U.S. and Japan. Rheumatic disease is still more common in underdeveloped countries.1
- Commonly occurs in children <5 yr (80%); peak incidence is in infants ages 6 to 11 mo. Although occurrence is rare after late childhood, the disease can occur in adolescence.1
- The highest incidence is found in Japan (∼369 cases/100,000 children <5 yr).2
- Incidence of KD in the U.S. is estimated to be 4 to 25 cases/100,000 children <5 yr of age.1
- Incidence in Western countries has largely stabilized, whereas incidence in East Asia continues to increase.
- Children of Asian or Pacific Islander descent have a higher incidence of KD compared with those of European or African descent, suggesting genetic susceptibility.3,4
- KD is more prevalent in males than females (1.5:1) and among younger children (80% of KD in children <5 yr and 50% in children under 2 yr).3
- Temporal clustering with winter and early spring predominance has been observed in KD in North America.3
- 1% of patients with KD in Japan have a positive family history of KD.2
- In Japan, the relative risk of KD in a sibling is 10-fold higher; half of these cases occur within 10 days of the initial case.2
- Single-nucleotide polymorphisms in six genes or gene regions have been implicated in susceptibility to KD.1,3
- Variants in transforming growth factor (TGF-β) signaling pathway genes have been associated with aneurysm risk in Europeans.1
- Reports from around the world have documented a spectrum of COVID-19 (SARS-CoV-2) presentation in children that is similar to KD, and termed COVID-19-associated KD and COVID-19-associated multisystem inflammatory syndrome in children (MIS-C).4-6
- The clinical presentation is characterized by a systemic inflammation of small- and medium-sized blood vessels.7
- There is no specific test that is diagnostic for KD. Confirmation of the diagnosis is based on clinical criteria.
- To meet the case definition of classic or typical KD, a child will have had fever persisting for ≥5 days and the presence of ≥4 of the following five principal features, which need not be present at the same time. The diagnosis may be made with only 4 days of fever in the presence of all five principal clinical criteria1,3,7:
- Bilateral, bulbar conjunctival injection with limbic sparing and without exudate.
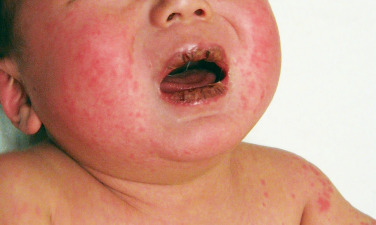
- Oral mucosal changes (Fig. E1): Erythema and fissured/cracking lips, strawberry tongue, diffuse injection of the oropharyngeal mucosa. Oral ulcers and pharyngeal exudates are not typical of KD.
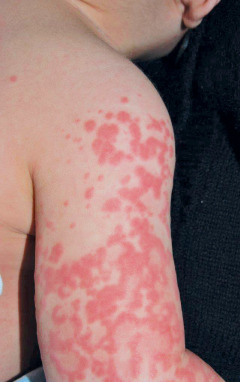
- Polymorphous exanthem (Fig. E2).
- Extremity changes: (a) Acute: Erythema and edema of hands and feet; (b) convalescent: Membranous desquamation of fingertips.
- Cervical lymph node enlargement (at least one lymph node ≥1.5 cm in diameter).
- Patients with unexplained fever ≥5 days and only two or three criteria are categorized as “incomplete” or “atypical KD,” but with evidence of coronary artery aneurysms these individuals are classified as complete KD. In infants <6 mo, atypical KD may present only as prolonged fever >7 days. These patients are more likely to be infants and older children and consequently are at higher risk for coronary aneurysms.1
- The fever of KD is mildly responsive to antipyretics and is usually >102.2° F (39° C) and often >104.0° F (40° C). If untreated, it lasts for 1 to 3 wk. Fever will typically resolve 36 h after treatment with intravenous immunoglobulin (IVIG).1,7
- The rash of KD can be maculopapular, diffuse erythroderma, or erythema multiforme-like; however, vesicles, bullae, purpura, and petechiae are never observed.1,7
- The pericardium, myocardium, endocardium, valves, and coronary arteries may be inflamed during acute illness. Cardiac abnormalities in KD are summarized in Box E2.1
- Valvular dysfunction occurs in ∼25% of patients regardless of coronary artery involvement and most often involves the mitral valve.1
- ∼5% of children with KD in the U.S. have cardiovascular collapse and shock at clinical presentation. Often, a diagnosis of bacterial sepsis is suspected at the outset, frequently with negative cultures and persistent fevers, in which case the diagnosis of KD should be suspected.1,3
- Coronary artery aneurysms can develop in as many as 25% of untreated children between 1 to 4 wk of illness. This subset of patients can develop myocardial infarction (MI) and congestive heart failure over time, which may be fatal.1,3
- New aneurysms seldom form after 6 wk. Half of the aneurysms show angiographic regression in 1 to 2 yr.1,3
- Coronary z-scores (coronary artery internal diameter normalized for body surface area) are the preferred method for describing coronary artery abnormalities and should be used to describe the left anterior descending artery and right coronary arteries over time.
- Morbidity and mortality rates are highest if the aneurysm has both a z-score ≥10 and an absolute dimension ≥8 mm (“giant aneurysms”).1
- Children with KD who are <1 yr or >6 yr are more likely to develop the cardiac sequelae and are least likely to respond to treatment.1
- Cervical lymphadenopathy is the most commonly absent physical manifestation in atypical KD, followed by exanthem and then extremity changes.3
- Oral mucosal changes are the most common manifestations of KD, affecting ∼90% of cases (either typical or atypical).1,7
- Sensitivity to light, uveitis, as well as nonexudative conjunctivitis may develop.7
- Redness and induration can be seen at the site of prior bacille Calmette-Guérin (BCG) inoculation.1,7
- Beau lines (transverse lines across the nails), diarrhea, acute myocarditis, cough, rhinorrhea, dyspnea, arthralgia, and myalgia may also be seen.1
- Aseptic meningitis can develop in 40% of cases.1
- Interstitial nephritis, acute renal failure in rare cases, KD shock syndrome, and macrophage activation syndrome can also occur.1
- Self-limiting arthritis involving large joints including knees, ankles, and hips was reported in 7.5% to 25% of patients, commonly in the second to third week of illness.1
- Associated noncardiac features of KD are summarized in Box E3.1
Courtesy Joseph F. Merola. From Callen JP et al: Dermatological signs of systemic disease, ed 5, Philadelphia, 2017, Elsevier.
Figure E2 Polymorphous, exanthematous eruption seen in a patient with Kawasaki disease.
Courtesy Joseph F. Merola. From Callen JP et al: Dermatological signs of systemic disease, ed 5, Philadelphia, 2017, Elsevier.
BOX E2 Cardiac Abnormalities in Kawasaki Disease
From Cherry JD et al: Feigin and Cherry’s pediatric infectious diseases, ed 8, Philadelphia, 2019, Elsevier.
- The cause of KD is still unknown despite decades of research.1
- Evidence suggests an infectious etiology precipitating an immune-mediated reaction in genetically susceptible individuals.2
- One hypothesis is that tropospheric winds from northeastern China carry the etiologic agent of KD from its source to Japan.
- SARS-CoV-2 epidemic causing COVID-19 is associated with high incidence of KD.5