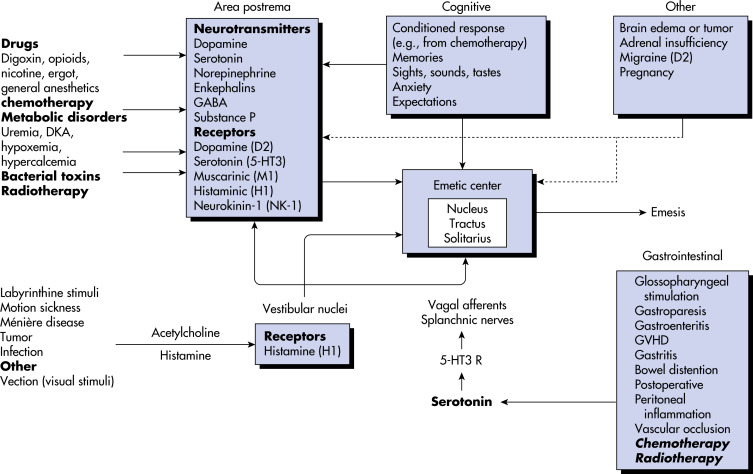
- A management approach to CINV is summarized in Box E1. Table E2 summarizes antiemetic drugs based on class.
- Recently, a proposed prediction tool for identifying patients at high-risk for CINV has listed eight risk factors:
- Patient age <60 yr
- The first two cycles of chemotherapy
- Anticipatory nausea and vomiting
- History of morning sickness
- Hours of sleep the night before chemotherapy
- CINV in the prior cycle
- Patient self-medication with nonprescribed treatments
- Use of platinum or anthracycline-based regimens
TABLE E2 Antiemetic Drugs
| Antiemetic Class | Mechanism of Action | Recommended Uses | Level of EvidenceTo Support Use inCancer-RelatedNausea and Vomiting |
|---|
Corticosteroid:
Dexamethasone | Unknown, possibly reduced prostaglandin activity in the brain | Treatment-related nausea and vomiting
Nausea and vomiting secondary to raised intracranial pressure | High |
| | Vomiting secondary to bowel obstruction | Low |
5-HT3 antagonist:
Granisetron
Ondansetron
Palonasetron
Dolasetron | Inhibit visceral afferents by blocking binding of peripheral 5-HT to serotonin (type 3) receptors along the vagus nerve in the GI tract | Treatment-related nausea and vomiting
Opioid-induced nausea and vomiting
Nausea of advanced cancer | High |
NK1 antagonist:
Aprepitant
Fosaprepitant | Blockade of the binding of substance P to NK1 receptors in the chemotrigger zone of the area postrema | Treatment-related nausea and vomiting | High |
Benzodiazepine:
Lorazepam
Alprazolam | Presumably due to anxiolytic, sedative and anterograde amnesic effects of these medications centrally
Especially useful in delayed or anticipatory nausea as adjuvant agents | Adjuvant support for the management of treatment-related nausea and vomiting, particularly when nausea associated with anxiety | High |
Atypical antipsychotic:
Olanzapine | Antipsychotic in the thienobenzodiazepine drug class that blocks multiple neurotransmitters, including:
- Dopamine at D1, D2, D3, and D4 brain receptors
- Serotonin at 5-HT2a, 5-HT2c, 5-HT3, and 5-HT6 receptors
- Catecholamines at alpha-1 adrenergic receptors
- Acetylcholine at muscarinic receptors
- Histamine at H1 receptors | Adjuvant agent to manage treatment-related nausea and vomiting | Moderate |
Antihistamine:
Diphenhydramine
Promethazine | Predominantly histamine antagonist in vestibular nucleus and chemotrigger zone
Central dopamine and acetylcholine antagonist | Adjuvant, especially when the extra-pyramidal effects of dopamine antagonists are considered problematic | Moderate |
Butyrophenone:
Haloperidol
Droperidol | Dopaminergic (D2 subtype) receptor antagonists | Adjuvant | Moderate |
Phenothiazine:
Prochlorperazine | Dopamine antagonist
Histamine antagonist
Acetylcholine antagonist | Adjuvant | High |
Dopamine2-antagonist:
Metoclopramide | Competitive antagonist at central dopaminergic (D2) receptors
Weak competitive antagonist peripherally at GI 5-HT3 receptors (at high dose)
5-HT4 agonist
Increased lower esophageal sphincter pressure
Enhanced rate of gastric emptying | Adjuvant
Gastric stasis
Opioid-induced nausea and vomiting
Nausea of advanced disease | High |
5-HT, 5-hydroxytryptamine; GI, gastrointestinal; NK1, neurokinin-1.
From Talley NJ et al: Essentials of internal medicine, ed 4, Chatswood, NSW, 2021, Elsevier Australia.
BOX E1 Management Approach: Chemotherapy-Induced Nausea and Vomiting (CINV)
Antiemetic therapy for CINV should be based on the emetic potential of the chemotherapy regimen being used and should take into account individual patient factors (e.g., sex, age, history of alcohol use, and previous emesis with chemotherapy). Excellent methods for predicting the likelihood of emesis with single chemotherapeutic agents or combination regimens have been developed. All patients receiving chemotherapy with moderate or high emetogenic potential should receive antiemetic prophylaxis for acute (day 1) and delayed (days 2-5) nausea and vomiting. Patients receiving highly emetogenic regimens should receive prophylaxis on day 1 with a 5-HT3 receptor antagonist (preferably palonosetron) plus neurokinin-1 (NK1) receptor antagonist plus dexamethasone, followed on days 2-4 by dexamethasone. Olanzapine should be added in patients judged to be at high risk. For moderately emetogenic regimens, treatment with a 5-HT3 receptor antagonist (preferably palonosetron) plus dexamethasone on day 1, followed by dexamethasone on days 2 and 3, is recommended. Patients receiving therapy with low emetic potential should receive dexamethasone (8 mg intravenously or orally); a 5-HT3 receptor antagonist should be added with subsequent courses only if antiemetic control is inadequate. Routine prophylaxis is not necessary for patients receiving agents or regimens of minimal risk. Patients with inadequate control should have intensification of their antiemetic prophylaxis during subsequent treatment cycles. |
From Niederhuber JE: Abeloff’s clinical oncology, ed 6, Philadelphia, 2020, Elsevier.
On a 32-point scale, calculated before each cycle of therapy, patients with risk scores ≥16 units are considered at high risk for developing moderate CINV.3
- Choice and duration of antiemetic use are dependent on the emesis-potential of the chemotherapy regimen being used. Commonly used antiemetic agents and recommended dosing are described in Table E3. For chemotherapy agents with high emetogenic potential, the use of multidrug antiemetic regimens has proven to be highly effective as prophylaxis.
- The most common treatment combination includes a serotonin-receptor (5-HT3) antagonist (ondansetron, granisetron, dolasetron, or palonosetron), a corticosteroid (dexamethasone), and a neurokinin-1 (NK-1) receptor antagonist (aprepitant, rolapitant, or fosaprepitant).4
- A fixed-dose combination of oral palonosetron and netupitant is now available and has the potential advantage of decreasing dexamethasone requirements for emesis control.
- Table E4 summarizes recommended antiemetic prophylaxis for patients receiving intravenous chemotherapy.
- The antipsychotic drug olanzapine has been demonstrated across multiple clinical trials to be effective and safe at reducing emesis during the delayed and overall phases of CINV prevention in highly-emetogenic chemotherapy regimens.
- Many other adjunct drugs are available, such as prochlorperazine, metoclopramide, haloperidol, and droanbinol; comparatively, they are less effective and have greater potential for adverse effects.
- Benzodiazepines (usually lorazepam) may help in patients with significant anxiety levels that lead to anticipatory CINV.
- Patients with uncontrolled symptoms may require hospitalization for supportive care including intravenous medications and fluids.
TABLE E3 Antiemetic Agents: Recommended Dosing
| Antiemetic Agent | RECOMMENDED DOSE |
|---|
| Acute Emesis (Before Chemotherapy) | Delayed Emesis |
|---|
| 5-HT3 antagonists |
| Ondansetron | 0.15 mg/kg or 8 mg IV; 12-16 mg PO | 8 mg PO twice a day × 2-3 days |
| Granisetron | 1 mg IV or PO; 10 mg subcutaneous (extended release) | |
| Dolasetron | 1.8 mg/kg or 100 mg IV; 100-200 mg PO | |
| Palonosetron | 0.25 mg IV or 0.5 mg PO | |
| NK1 receptor antagonists |
| Aprepitant | 125 mg PO | 80 mg PO days 2 and 3 |
| Fosaprepitant | 150 mg IV | |
| Rolapitant | 180 mg PO | |
| Combination 5-HT3/NK1 receptor antagonist |
| NEPA (palonosetron 0.5 mg/netupitant 300 mg) | 1 tablet PO | |
| Multireceptor antagonist |
| Olanzapine | 10 mg PO | 10 mg PO days 2-4 |
| Corticosteroids |
| Dexamethasone |
| With NK1 antagonist | 12 mg IV or PO | 8 mg PO for 2-3 days |
| Without NK1 antagonist | 8 mg (moderate risk) or 20 mg (high risk) IV or PO | 4-8 mg PO twice a day for 2-3 days |
| Other agents |
| Prochlorperazine | | 10 mg PO or IV every 3-4 h as needed |
| Lorazepam | 1-2 mg IV (for anticipatory nausea/vomiting) | |
| Dronabinol | | 5 mg/m2 PO every 3-4 h as needed |
5-HT3, 5-Hydroxytryptamine 3; NK1, neurokinin 1; IV, intravenous; PO, by mouth.
From Niederhuber JE: Abeloff’s clinical oncology, ed 6, Philadelphia, 2020, Elsevier.
TABLE E4 Summary of Recommended Antiemetic Prophylaxis for Patients Receiving Intravenous Chemotherapy
| Risk of Emesis | Day 1 (Prechemotherapy) | After Day 1 |
|---|
| High (includes cyclophosphamide and doxorubicin) | 5-HT3 receptor antagonist (palonosetron preferred) + NK1 receptor antagonist + dexamethasone + olanzapine (if patient considered high risk) | Dexamethasone days 2-4 + aprepitant days 2-3 (if used on day 1) + olanzapine days 2-4 (high-risk patients) |
| Moderate | 5-HT3 receptor antagonist (palonosetron preferred) + dexamethasone | Dexamethasone days 2 and 3 |
| Low | Dexamethasone | None |
| Minimal | As needed | None |
5-HT3, 5-Hydroxytryptamine 3; NK1, neurokinin 1.
From Niederhuber JE: Abeloff’s clinical oncology, ed 6, Philadelphia, 2020, Elsevier.
Nonpharmacologic TherapyPatients who have a significant anxiety component to their CINV may benefit from cognitive behavioral therapy.
DispositionCINV treatment has been revolutionized with the introduction of newer classes of antiemetics leading to a significant majority of patients achieving adequate symptom control.