AUTHOR: Alexei Shimanovsky, MD
A gastrinoma is a neuroendocrine neoplasm (NEN) characterized by the secretion of gastrin with a resultant hypergastrinemic state resulting in gastroesophageal reflux disease, severe recurrent peptic ulcer disease, and chronic diarrhea. This condition has been used synonymously with Zollinger-Ellison syndrome (ZES); however, gastrinoma refers to the NEN secreting gastrin, whereas ZES refers to the disease.1,2
Zollinger-Ellison (ZE) syndrome
| ||||||||||||||||
- Incidence is approximately 1 to 1.5 cases per million per year.
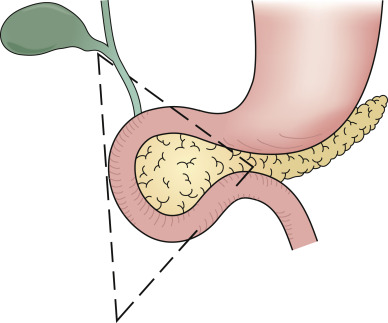
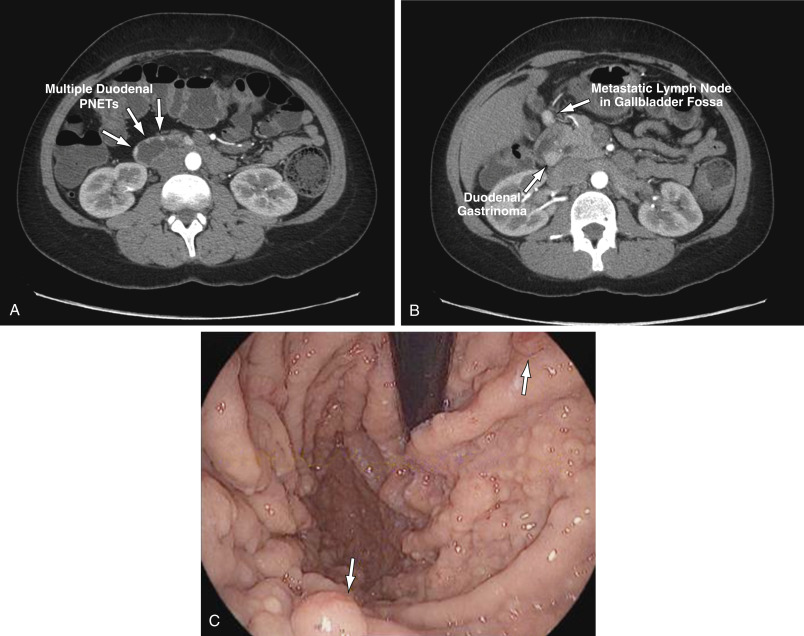
- Gastrinomas are NENs located in the duodenum (70%), pancreas (20%), and rarely in other sites (10%), such as lung, stomach, liver, and ovary. Ninety percent of gastrinomas are located within the “gastrinoma triangle,” bounded by lines connecting the cystic duct, the junction between the second and third portions of the duodenum, and the junction between the neck and body of the pancreas (Fig. E1).
- Females have a slightly greater preponderance for developing the disease, and gastrinoma is most frequently diagnosed between the ages of 20 and 50 yr.
- Two thirds of gastrinomas are sporadic, and one third are associated with multiple endocrine neoplasia type 1 (MEN-1), an autosomal-dominant genetic disorder that also includes hyperparathyroidism and pituitary tumors.
- Approximately 60% of gastrinomas are malignant.
- The incidence and prevalence of pancreatic neuroendocrine tumors are increasing. They represent 1.3% of all cases of pancreatic cancer.
The Triangle is Bounded by the Lines Connecting the Cystic Duct, the Junction Between the Second and Third Portions of the Duodenum, and the Junction Between the Neck and Body of the Pancreas.
From Townsend CM et al: Sabiston textbook of surgery, ed 21, St Louis, 2022, Elsevier.
The diagnosis of ZES is not always simple and timely due to nonspecific symptoms and factors such as medications (e.g., proton pump inhibitors), which may hide the ongoing symptoms.
- The majority of patients (95%) present with symptoms of peptic ulcer (see Section I, “Peptic Ulcer Disease”).
- 60% of patients have symptoms related to gastroesophageal reflux disease (see Section I, “Gastroesophageal Reflux Disease”).
- One third of patients with ZES have diarrhea and, less commonly, steatorrhea.
The following circumstances warrant suspicion of ZES:
- Ulcers distal to the first portion of the duodenum
- Multiple peptic ulcers
- Ineffective treatment for peptic ulcer disease with the usual drug doses and schedules
- Peptic ulcer and diarrhea
- Familial history of peptic ulcer
- Patients with a personal or family history suggesting parathyroid or pituitary tumors or dysfunction
- Peptic ulcer and urinary tract calculi
- Patients with peptic ulcer who are negative for Helicobacter pylori and do not have a history of nonsteroidal antiinflammatory drug use
- Gastrinomas are derived from the enteroendocrine cells that arise from the embryologic endoderm and form tumors mainly in the pancreas but also in the proximal small intestine. They are classified as well-differentiated neuroendocrine tumors (NETs).
- The pathophysiologic manifestations of ZES are related to the effects of hypergastrinemia. Gastrin stimulates gastric acid secretion, which in turn is responsible for the development of duodenal ulcers and diarrhea. Gastrin also promotes gastric mucosal epithelial cell growth and resulting parietal cell hyperplasia.
- Gastrinomas are usually small (0.1 to 2 cm) but sometimes large (>20 cm) tumors.
- 60% of gastrinomas are malignant, with liver and regional lymph nodes the most common site of metastases. Histology is not a good predictor of the biology of gastrinomas.
- 60% of patients with MEN-1 have gastrinomas.
- 10% of patients with ZES have islet cell hyperplasia rather than gastrinomas; in 10% to 20% of patients, tumors cannot be located because of their small size.