AUTHORS: Javad Najjar Mojarrab, MD, MBA and James E. Novak, MD, PhD
Hepatorenal syndrome (HRS) is a functional cause of acute kidney injury (AKI) that occurs in the setting of severe acute or chronic liver disease, most commonly with cirrhosis and portal hypertension. HRS develops because of reduced renal perfusion caused by hemodynamic alterations in the arterial circulation and overactivity of endogenous vasoactive systems.
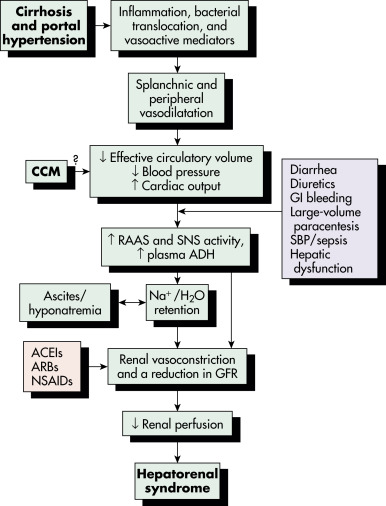
In HRS, liver dysfunction leads to increased production or activity of vasodilators such as nitric oxide. Vasodilation is most pronounced in the splanchnic circulation and, combined with cardiac dysfunction, results in systemic hypotension. Disturbed vasoconstrictor-vasodilator balance in the kidney favors vasoconstriction. Intense renal arteriolar vasoconstriction, renal cortical hypoperfusion, and AKI ensue (Fig. E1). Fig. E2 illustrates the proposed pathophysiology of HRS and HRS precipitants. HRS involves not only circulatory dysfunction but also systemic inflammation triggered by hepatic injury and bacterial translocation from the gut lumen.
There are two types of HRS (Table E1). The International Club of Ascites (ICA) revised the nomenclature and diagnosis of type 1 HRS (HRS-1), now termed HRS-acute kidney injury (HRS-AKI), and type 2 HRS (HRS-2), also now termed HRS-nonacute kidney injury (HRS-NAKI).
- HRS-AKI: Acute and rapid deterioration in kidney function (Table E2)
- HRS-NAKI: Moderate stable chronic kidney disease (CKD) (average serum creatinine [SCr] 1.5 mg/dl [133 mmol/L])
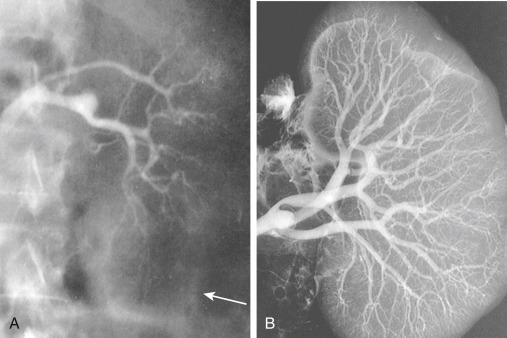
Figure E1 Circulatory dysfunction in hepatorenal syndrome (HRS).
A, Renal angiogram (the arrow marks edge of the kidney). B, The angiogram carried out in the same kidney at autopsy. Note complete filling of the renal arterial system throughout the vascular bed to the periphery of the cortex. The vascular attenuation and tortuosity seen previously (A) are no longer present. The vessels are also histologically normal. This finding indicates the functional nature of the vascular abnormality in HRS.
From Floege J et al: Comprehensive clinical nephrology, ed 4, St Louis, 2010, Saunders.
Figure E2 Proposed pathophysiology and triggers of hepatorenal syndrome.

ACEI, Angiotensin-converting enzyme inhibitor; ADH, antidiuretic hormone; ARB, angiotensin receptor blocker; CCM, cirrhotic cardiomyopathy; GFR, glomerular filtration rate; GI, gastrointestinal; H2O, water; NA, sodium; NSAID, nonsteroidal antiinflammatory drug; RAAS, renin-angiotensin-aldosterone system; SBP, spontaneous bacterial peritonitis; SNS, sympathetic nervous system.
From Feldman M et al: Sleisenger and Fordtran’s gastrointestinal and liver disease, ed 10, Philadelphia, 2016, Elsevier.
TABLE E1 Definition of Hepatorenal Syndrome
| HRS-AKI (Type 1) | |||
| Acute and rapid deterioration in kidney function: An acute increase in serum creatinine of ≥0.3 mg/dl within 48 hr and/or urinary output <0.5 ml/kg for >6 hr or increase in serum creatinine of ≥50% from a stable baseline serum creatinine within 3 mo (presumed to have developed within the past 7 days when no prior readings are available) Occurs in parallel with the cirrhosis-associated failure of other organs or systems (e.g., coagulopathy, hepatic encephalopathy) In cirrhosis, HRS is a form of acute-on-chronic liver failure Frequently follows a precipitating event, mainly bacterial infection; rapidly fatal without treatment Renal replacement therapy, i.e., hemodialysis, does not prolong life without liver transplantation | |||
| HRS-NAKI (Type 2) | |||
| HRS-AKD | Hepatorenal syndrome-acute kidney disease: (a) eGFR <60 ml/min per 1.73 m2 for <3 mo in the absence of other (structural) causes; or (b) SCr increase <50% using the last available outpatient value within 3 mo | ||
| HRS-CKD | Hepatorenal syndrome-chronic kidney disease: eGFR <60 ml/min per 1.73 m2 for 3 mo in the absence of other (structural) causes | ||
| Moderate stable CKD Mean survival of 6 mo | |||
AKI, Acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HRS, hepatorenal syndrome; NAKI, nonacute kidney injury; SCr, serum creatinine.
From Fernández J, Arroyo V: Hepatorenal syndrome. In Johnson RJ et al (eds): Comprehensive clinical nephrology, ed 5, Philadelphia, 2015, Saunders.
TABLE E2 Hepatorenal Syndrome-AKI Classified by Acute Kidney Injury (AKI) Stage According to the International Club of Ascites and Acute Kidney Injury Network Criteria
| Risk/Stage 1 | Increase in SCr >1.5- to 2-fold from baseline; or by >0.3 mg/dl (26.5 mmol/L) Increase in SCr of 50% or more to a final value of >1.5 mg/dl (133 mmol/L) | ||
| Injury/Stage 2 | Increase in SCr >2- to 3-fold from baseline | ||
| Failure/Stage 3 | Increase in SCr >3-fold from baseline; or SCr >4 mg/dl (353.6 mmol/L) with an acute increase of ≥0.5 mg/dl (44 mmol/L); or initiation of renal replacement therapy |
SCr, Serum creatinine.
From Angeli P et al: Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites, J Hepatol 62:968, 2015.
Oliguric renal failure of cirrhosis
| ||||||||||||||||
The probability of HRS in patients with cirrhosis is 18% at 1 yr and 39% at 5 yr. HRS is associated with poor prognosis. HRS-AKI is associated with >90% mortality in 3 mo, with higher mortality conferred by a Model for End-stage Liver Disease (MELD) score >30 and by higher stages of AKI. HRS-NAKI is associated with 30% mortality at 3 mo and 60% at 1 yr. Although the cause of liver failure or MELD score does not predict the development of HRS, MELD score predicts mortality when HRS has occurred. Hyponatremia is an independent risk factor for HRS and is associated with elevated renin levels, which are thought to reflect the overactivated neurohumoral response.
There are no specific physical findings associated with HRS, although it is usually associated with signs and symptoms of acute or chronic decompensated liver failure, such as hypotension, jaundice, spider angiomata, splenomegaly, ascites, fetor hepaticus, edema, asterixis, encephalopathy, or coma. Usually, HRS is accompanied by oliguria and bland urine sediment. Urine chemistries tend to show a low rate of urinary sodium excretion (usually <10 mEq/L). However, a nonoliguric state or an active urine sediment (blood, casts, and/or protein) does not exclude the diagnosis of HRS.
Precipitating events are identified in most cases of HRS. Contributory factors include bacterial infection, alcoholic hepatitis, excessive laxative or NSAID use, large-volume paracentesis without albumin administration, Gl bleeding, or a major surgical procedure. Unlike HRS, prerenal azotemia improves with cessation of diuretic therapy and/or volume resuscitation. Nevertheless, HRS may present in the absence of a clear precipitating factor. Recent studies suggest that, among those not listed for liver transplant, mortality rates are extremely high regardless of whether the etiology of AKI is HRS or acute tubular necrosis (ATN).