AUTHOR: Glenn G. Fort, MD, MPH



DefinitionDiabetic foot infections (DFIs) are a common and potentially serious problem in persons with diabetes. They usually arise from either a skin ulceration that occurs secondarily to peripheral neuropathy or in a wound caused by some form of trauma. The infection usually involves one or more bacteria and can spread to contiguous tissues including bone, causing an osteomyelitis.
SynonymsDiabetic foot ulcer
Diabetic foot infection
DFI
| ICD-10CM CODES | | E11.621 | Type 2 diabetes mellitus with foot ulcer | | E10.5 | Diabetes mellitus with peripheral circulatory complications | | E10.6D | Diabetes mellitus with other specific complications |
|
Epidemiology & DemographicsIncidenceDFIs are the most common cause of hospitalizations for diabetic patients. They account for 20% of all hospital admissions. Nearly one in six patients will die within 1 yr of their infection.
Prevalence25 million people in the U.S. have diabetes, of which 19% to 34% will develop a foot ulcer in their lifetime, and more than 50% of these will become infected.
Predominant Sex & AgeFemales greater than males
Peak IncidenceMore common in Hispanics, African Americans, and Native Americans due to increased rates of diabetes in those populations
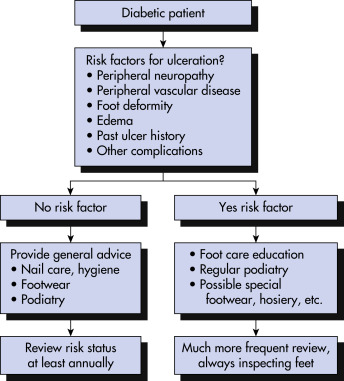
Risk Factors
- Diabetes greater than 10 yr
- Poor glucose control
- Peripheral neuropathy: Altered protective sensation and altered pain response
- Diabetic angiopathy: Atherosclerotic obstruction of larger vessels leading to peripheral vascular disease
- Evidence of increased local pressure: Callus or erythema
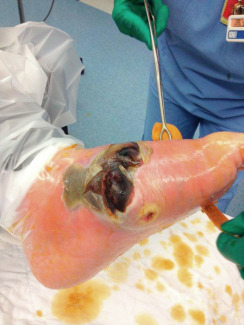
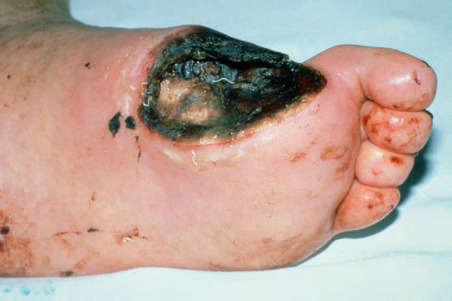
Physical Findings & Clinical Presentation
- Based on guidelines by Infectious Diseases Society of America, infection is present if obvious purulent drainage and/or the presence of two or more signs of inflammation:
- Erythema
- Pain
- Tenderness
- Warmth
- Induration
- Systemic signs of infection include:
- Anorexia, nausea/vomiting
- Fever, chills, night sweats
- Change in mental status and recent worsening of glycemic control
- An earlier and commonly used classification system was originally proposed by Wagner (Table 1).
- An update to the Wagner system was introduced at the University of Texas (UT), San Antonio (Table 2), U.S. While similar to Wagner in its first three categories, this later system eliminated grades 4 and 5 and added stages A-D for each of the grades. The UT system was the first diabetic foot ulcer classification to be validated. University of Texas system Grade:
- Grade 0: Pre- or postulcerative (Stages A to D)
- Grade 1: Full-thickness ulcer not involving tendon, capsule, or bone (Stages A to D)
- Grade 2: Tendon or capsular involvement without bone palpable (Stages A to D)
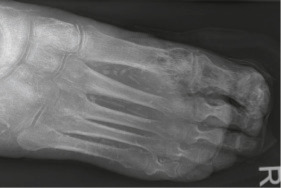
- Grade 3: Probes to bone (Stages A to D)
TABLE 2 University of Texas Wound Classification System
| Stage | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|
| A | Preulcer or postulcer lesion; no skin break | Superficial ulcer | Deep ulcer to tendon or capsule | Wound penetrating bone or joint |
| B | + Infection | + Infection | + Infection | + Infection |
| C | + Ischemia | + Ischemia | + Ischemia | + Ischemia |
| D | + Infection and ischemia | + Infection and ischemia | + Infection and ischemia | + Infection and ischemia |
Modified from Armstrong DG et al: Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation, Diabetes Care 12:855-859, 1998. In Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2020, Elsevier.
TABLE 1 Wagner Diabetic Foot Ulcer Classification System
| Grade | Description |
|---|
| 0 | No ulcer, but high-risk foot (e.g., deformity, callus, insensitivity) |
| 1 | Superficial full-thickness ulcer |
| 2 | Deeper ulcer, penetrating tendons, no bone involvement |
| 3 | Deeper ulcer with bone involvement, osteitis |
| 4 | Partial gangrene (e.g., toes, forefoot) |
| 5 | Gangrene of whole foot |
Modified from Oyibo S et al: A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems, Diabetes Care 24:84-88, 2001. In Melmed S et al: Williams textbook of endocrinology, ed 14, Philadelphia, 2020, Elsevier.
Stage:
- A: Noninfected
- B: Infected
- C: Ischemic
- D: Infected and ischemic
Etiology
- Most diabetic foot infections are polymicrobial (can involve five to seven different bacteria) and depend on the extent of involvement.
- Superficial infections are likely due to gram-positive skin bacteria:
- Staphylococcus aureus, includes methicillin-resistant S. aureus (MRSA)
- Streptococcus agalactiae (group B streptococcus) and Streptococcus pyogenes (group A streptococcus)
- Coagulase-negative Staphylococcus
- Infections that are deep, chronically infected, or previously treated are likely to be polymicrobial:
- Include above bacteria plus enterococci, gram-negative rods including Pseudomonas aeruginosa and anaerobes
- With gangrene, can expect more anaerobic bacteria such as Clostridia and Bacteroides species
- Patients with multiple admissions can have more resistant bacteria such as ESBL-type resistant gram-negative rod bacteria, MRSA, and Acinetobacter

Empiric antibiotic regimen should be started based on likely pathogens suspected and severity of disease. Wound management and debridement including surgical consultation are important as well.
Nonpharmacologic Therapy
- Good nutrition will promote wound healing.
- Glycemic control will promote healing.
- Fluid and electrolyte balance will improve healing.
Acute General TreatmentWound Management
- Debridement of callus and necrotic tissues by wound care specialist or surgeon and at times may require multiple debridements.
- Wound dressing: To absorb exudates and promote healing. Many products are available, but none has been proven superior and include:
- Enzymes
- Gels
- Hydrocolloids
- Antiseptics containing iodine or silver salts
- Honey
- Relieve pressure on the foot: Casts or special shoes.
- Amputation or revascularization procedures such as angioplasty or bypass grafting may be necessary.
Antibiotic Management
- Prior to receiving culture results an empiric antibiotic regimen should be started as soon as possible to cover skin bacteria, gram-negative rods, and anaerobes. Options for intravenous therapy include:
- Piperacillin-tazobactam: 3.375 g IV q6h with normal kidney function. Will cover gram-negative rods including Pseudomonas aeruginosa, streptococci, anaerobes, and Staphylococcus aureus. Adjust dose based on CrCl.
- Meropenem: 1 g IV q8h with normal kidney function has comparable coverage as piperacillin-tazobactam. Similar agents include imipenem and doripenem.
- Third-generation cephalosporin such as cefepime, 2 g IV q8h, or ceftriaxone, 2 g IV qd, have excellent gram-negative coverage, and for anaerobic coverage add metronidazole, 500 mg IV q8h, or clindamycin 900 mg IV q8h. Cefepime will cover Pseudomonas aeruginosa, but ceftriaxone will not.
- For penicillin-allergic patients a combination of ciprofloxacin, 400 mg IV q12h, plus metronidazole or clindamycin is an option. Aztreonam is another option for gram-negative rod coverage, 2 g IV q8h.
- If MRSA is suspected, need to add IV vancomycin, 15 to 20 mg/kg IV q8 to 12h, depending on age and CrCl and follow through levels to keep above 15. Other options include daptomycin, 4 mg/kg IV qd, which does not have to be adjusted for CrCl, or linezolid, 400 to 600 mg IV q12h.
- If VRE is suspected, options include tigecycline, 100-mg IV load dose, then 50-mg IV q12h, which also covers MRSA and gram-negative rods but not Pseudomonas aeruginosa or can use daptomycin or linezolid.
- If ESBL gram-negative bacteria are suspected, then options include meropenem or ertapenem, 1 g IV qd.
- Once culture results are known, can tailor antibiotics to more specific agent.
- Oral antibiotics used for milder infections include amoxicillin-clavulanate, 875 mg PO q12h, which will cover gram-negative rods, streptococci, and anaerobes, or ciprofloxacin plus metronidazole or clindamycin. Bactrim will cover MRSA and MSSA and some gram-negative rods.
The expert panel on diabetic foot infection (DFI) of the International Working Group on the Diabetic Foot conducted a systematic review. Results of comparisons of different antibiotic regimens generally demonstrated that newly introduced antibiotic regimens appeared to be as effective as conventional therapy.
Chronic Treatment
- Length of therapy: Highly variable depending on the severity of the infection. In general, 2 to 4 wk of antibiotics is sufficient. If bone infection suspected or documented, may need 4 to 8 wk of antibiotics, preferably intravenous via a peripherally inserted central line (PICC line).
- Surgical debridement may also be necessary for several weeks.
Complementary Medicine
- Hyperbaric oxygen (HBO): Used as an adjunct to antibiotics, debridement, and revascularization in the therapy of chronic, nonhealing wounds associated with diabetes. Evidence of effectiveness is conflicting. HBO acts by:
- Inducing vasoconstriction and reducing vasogenic edema
- Facilitating fibroblast activity, angiogenesis, and wound healing
- Killing anaerobic bacteria and augmenting neutrophil bactericidal activity
- Negative pressure wound therapy (wound vac): Controlled, subatmospheric pressure applied to an open wound can accelerate healing and closure.
- An open cell foam insert is cut to fit the open wound and then secured under a clear, vapor-permeable, plastic dressing.
- Tubing extends from the sponge to a disposable collection canister.
- A portable pump applies 125 mm Hg of controlled suction to the system. The subatmospheric pressure (suction) is equally distributed across the open wound and evacuates stagnant fluid from the wound.
Disposition
- Following up on sed rates, CRP, BUN/CR, and levels of vancomycin if that antibiotic used.
- Surgical or wound center care follow-up.
- HBO usually involves multiple sessions over several weeks.
- Wound vac is applied for weeks and requires periodic nursing follow-up.
- The risk of death at 5 yr for a patient with a diabetic foot ulcer is two to five times as high as the risk for a patient with diabetes without a foot ulcer.
Referral
- Infectious disease consultant for antibiotic management
- Surgeon or wound care center for surgical treatments
- Endocrinologist for good diabetes care
- Vascular surgeon for angioplasty or bypass procedures