AUTHORS: Tara C. Bouton, MD, MPH, TM and Glenn G. Fort, MD, MPH
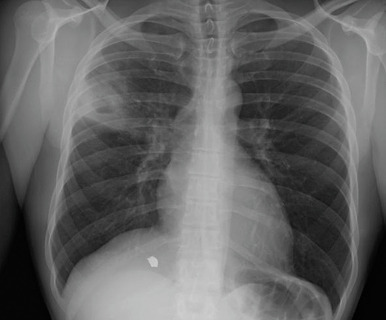
Pulmonary tuberculosis (TB) is an infection of the lung and, occasionally, surrounding structures, caused by the bacterium Mycobacterium tuberculosis (Mtb). Two states of M. tuberculosis infection are recognized: Latent tuberculosis infection (LTBI) and acute tuberculosis disease, although infection and immunologic control exist across a spectrum. LTBI is a state of persistent immune response to stimulation by M. tuberculosis antigens without evidence of clinically manifested active TB and with bacillary replication absent or below some undefined threshold as a result of immunologic control. Most persons with LTBI never become sick with TB; however, 5% to 15% have progression to tuberculosis disease.1 Multidrug-resistant (MDR) TB is defined as disease caused by strains of Mtb that are at least resistant to treatment with isoniazid (INH) and rifampin (RIF) (two of the most effective first-line drugs); extensively drug-resistant (XDR) TB refers to disease caused by MDR strains that are also resistant to treatment with any fluoroquinolone and bedaquiline or linezolid.
- One fourth of the world’s population is infected with TB, and it is one of the world’s leading infectious disease killers.
- In 2017, there were an estimated 10 million new cases of active TB; 9% involved coinfection with HIV; 1.3 million deaths from TB, including 300,000 among HIV-infected patients; 457,560 incident cases of multidrug-resistant TB worldwide.
- In 2018, a total of 9029 new TB cases were reported in the U.S., representing a 0.7% decrease from 2017. The U.S. TB incidence in 2018 was 2.8/100,000 persons.
- The rate among non-U.S.-born persons was >14 times that in the U.S.-born persons. Since 1993, TB case counts and rates have declined in the U.S. As the number of cases decreases overall, an increasing percentage of cases occurs among non-U.S.-born persons.
- More than 90% of new cases each year from reactivated prior infections, and 9% represent new infections.
- Only 10% of patients with purified protein derivative (PPD) conversions will develop TB, most within 1 to 2 yr, though this is higher in HIV-positive patients (8%/yr).
- Two thirds of new TB cases in 2018 occurred in non-U.S.-born persons, for whom the top five countries of birth were Mexico, the Philippines, India, Vietnam, and China.
- In 2017 in the U.S., 1.9% of cases were MDR TB, which is a decline from 8.2% in 2008. There were three cases of XDR TB in the U.S. in 2017.
- See “Etiology”
- Primary pulmonary TB infection generally asymptomatic
- Reactivation pulmonary TB:
- Progressive primary pulmonary TB disease: Same as reactivation pulmonary TB
- TB pleurisy:
- Rare massive, suffocating, fatal hemoptysis secondary to erosion of pulmonary artery within a cavity (Rasmussen aneurysm)
- Chest examination:
- Mtb, a slow-growing, aerobic, nonspore-forming, nonmotile bacillus, with a lipid-rich cell wall:
- Lacks pigment
- Produces niacin
- Reduces nitrate
- Produces heat-labile catalase
- Mtb staining, acid-fast and acid-alcohol fast by Ziehl-Neelsen method, appearing as red, slightly bent, beaded rods 2 to 4 microns long (acid-fast bacilli [AFB]), against a blue background
- Polymerase chain reaction (PCR) to detect <10 organisms/ml in sputum (compared with the requisite 10,000 organisms/ml for AFB smear detection)
- Culture:
- Genome sequencing:
- Humans are the only reservoir for Mtb
- Transmission:
- Aerosolized droplets containing AFB generated through cough, speaking, singing, bronchoscopy, or autopsy are inhaled directly into alveoli
- Facilitated by close exposure to high-velocity cough (unprotected by proper mask or respirators) from patients with AFB-positive sputum and cavitary lesions
- Locations of increased TB transmission risk include hospitals, homeless shelters, correctional facilities, nursing homes, and residential homes for those with HIV
- Pathogenesis:
- Mtb are ingested by macrophages in alveoli, then transported to regional lymph nodes, where spread is contained
- Some Mtb may reach bloodstream and disseminate widely
- Primary TB (often asymptomatic, minimal pneumonitis in lower or midlung fields, with hilar lymphadenopathy) is essentially an intracellular infection, with multiplication of organisms continuing 2 to 12 wk after primary exposure, until cell-mediated hypersensitivity (detected by positive skin test reaction to tuberculin PPD) matures, and results in subsequent containment of infection
- Local and disseminated Mtb are thus contained by the following T-cell-mediated immune responses:
- Recruitment of monocytes
- Transformation of lymphocytes with secretion of lymphokines
- Activation of macrophages and histiocytes
- Organization into granulomas, where Mtb may survive within macrophages (Langhans giant cells), but within which multiplication essentially ceases (95%) and from which spread is prohibited
- Progressive primary pulmonary disease:
- Postprimary TB pleurisy with pleural effusion:
- Develops after early primary infection, although often before conversion to positive PPD
- Results from pleural seeding from a peripheral lung lesion or rupture of lymph node into pleural space
- May produce a large (sometimes hemorrhagic) exudative effusion (with polymorphonuclear cells early, rapidly replaced by lymphocytes), frequently without pulmonary infiltrates
- Generally resolves without treatment
- Portends a high risk of subsequent clinical disease, and therefore must be diagnosed and treated early (pleural biopsy and culture) to prevent future catastrophic TB illness
- May result in disseminated extrapulmonary infection
- Reactivation pulmonary TB:
- Occurs months to years after primary TB
- Preferentially involves the apical posterior segments of the upper lobes and superior segments of the lower lobes
- Associated with necrosis and cavitation of involved lung, hemoptysis, chronic fever, night sweats, weight loss
- Spread within lung occurs via cough and inhalation
- Reinfection TB:
- Mtb in both progressive primary and reactivation pulmonary TB:
- Intracellular (macrophage) lesions (undergoing slow multiplication)
- Closed caseous lesions (undergoing slow multiplication)
- Extracellular, open cavities (undergoing rapid multiplication)
- INH and RIF are bactericidal in all three sites
- Pyrazinamide (PZA) especially active within acidic macrophage environment
- Extrapulmonary reactivation disease also possible
- Rapid local progression and dissemination in infants with devastating illness before PPD conversion occurs
- Most symptoms (fever, weight loss, anorexia) and tissue destruction (caseous necrosis) from cytokines and cell-mediated immune responses
- Mtb has no important endotoxins or exotoxins
- Granuloma formation related to tumor necrosis factor (TNF) secreted by activated macrophages