AUTHORS: Amy L. Bellinghausen, MD and Angela Wang, MD
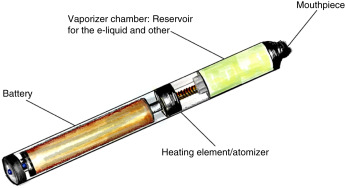
Electronic cigarette (e-cigarette) or vaping-associated lung injury (EVALI) refers to an acute lung injury due to inhalation of vaporized liquid, which contains nicotine or cannabis products, with or without added flavorings. Electronic nicotine and cannabis delivery systems are a diverse group of devices that are structurally engineered to aerosolize a liquid, which is then inhaled, or “vaped.” They include a variety of products, including e-cigarettes, vapes, vape pens, and hookah pens. Each device contains four compartments: A battery, a reservoir with liquid formulation, and a vaporizing chamber with a heating element, along with a mouthpiece for inhalation (Fig. E1). The acute lung injury associated with the use of these products is heterogenous, with variable severity, presentation, and duration.
E-cigarette or vaping product use-associated lung injury
(E-cigarette or) vaping-associated pulmonary injury; (E)VAPI
Vaping (product use)-associated lung injury; VALI
“Popcorn lung”-referring specifically to lung injury caused by diacetyl-containing flavor additives
| ||||||||||||||||||||||||||||||||
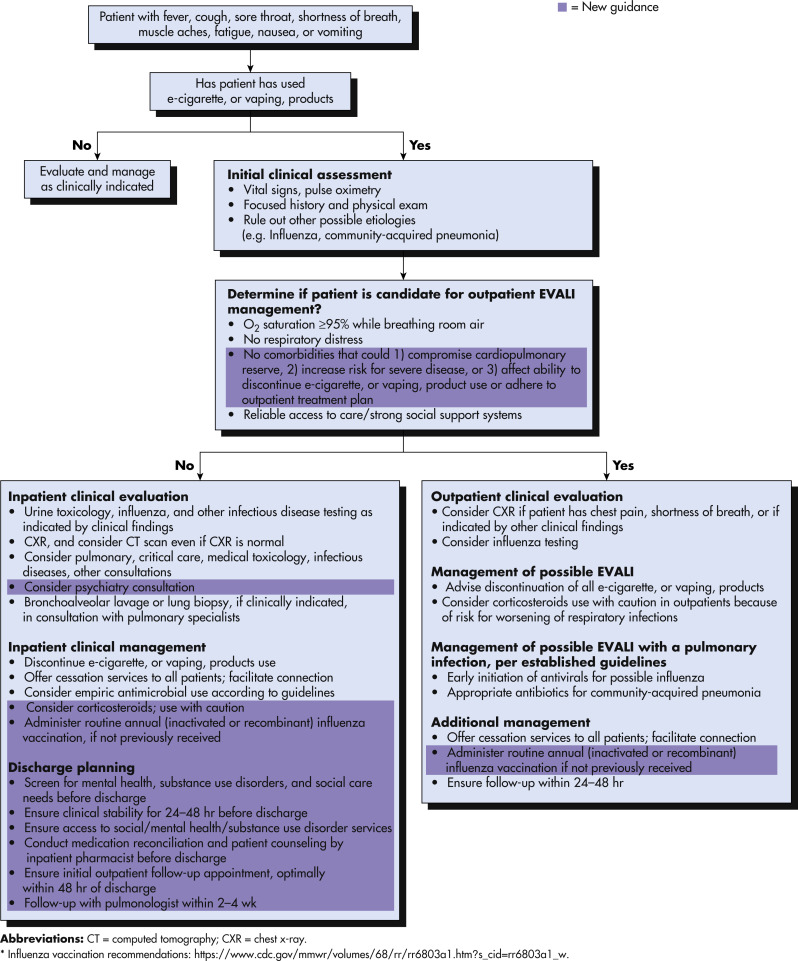
Because of the overlap in symptoms between EVALI and other acute causes of respiratory failure (particularly COVID-19), exact incidence rates of EVALI are difficult to ascertain. As of February 18, 2020, a total of 2807 hospitalized EVALI cases or deaths had been reported to the Centers for Disease Control and Prevention (case reporting of EVALI was stopped at that time, due to the emerging COVID-19 pandemic).1
The incidence of known EVALI cases increased significantly in August 2019 and peaked during September 2019.3 The subsequent COVID-19 pandemic has made identification of EVALI cases more challenging, but there have been no signs of a second rise in EVALI incidence.
Ethnic and racial minorities appear to be at greater risk. Although the majority of EVALI patients have been non-Hispanic white individuals, ethnic minorities are overrepresented compared with their baseline frequencies of vaping-product use.6
- Patients with EVALI may present with symptoms ranging from mild dyspnea to immediately life-threatening respiratory failure. Milder cases generally present with cough, shortness of breath, and dyspnea on exertion. Chest pain and fever are frequently encountered complaints. Patients may also report chills and gastrointestinal symptoms (diarrhea, nausea, and vomiting). Sputum production is less frequent (36% in one case series) and hemoptysis relatively uncommon. More severe cases are marked by profound dyspnea, tachycardia, tachypnea, and hypoxemia.7
- Laboratory abnormalities are nonspecific, with elevated leukocyte count, C-reactive protein, erythrocyte sedimentation rate, and procalcitonin. Peripheral eosinophilia is not generally found in EVALI cases.
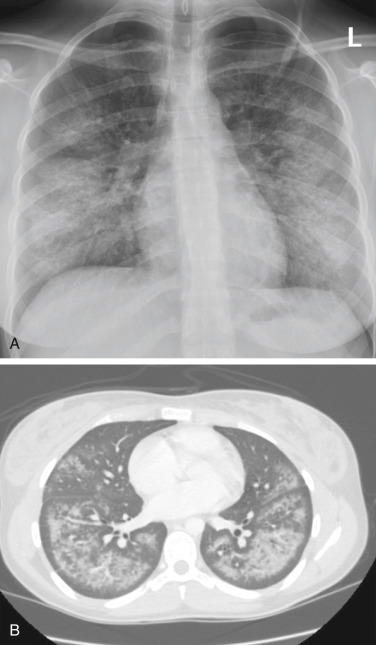
- Chest imaging findings are also nonspecific, but generally consist of patchy, ground glass opacities, occasionally with subpleural sparing seen on CT scans.
- Although EVALI generally presents with a diffuse alveolar damage phenotype, cases have been reported with more varied presentations, including hypersensitivity pneumonitis, acute eosinophilic pneumonia, organizing pneumonia, and lipoid pneumonia.
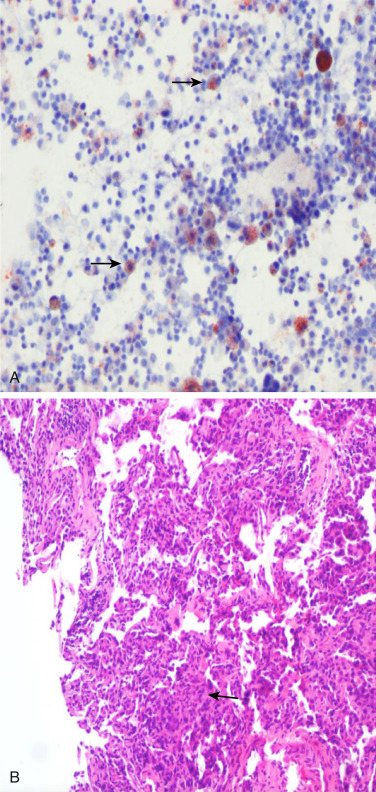
- The etiology of EVALI is not fully understood; several mechanisms have been proposed, including direct toxicity of e-liquid components causing lung epithelial necrosis and alteration of the inflammatory state of the lung by increasing proinflammatory cytokine release by macrophages (Fig. E2).
- Vitamin E (tocopherol acetate) is the causative compound most consistently found in bronchoalveolar lavage (BAL) fluid of patients with EVALI and in the vaping products used.8 Vitamin E is thought to have a direct toxic effect on pulmonary epithelium.
B, H:E Stain Showing Multifocal Interstitial Lymphocytic Inflammation (Arrow) (200×).
From Cherian SV et al: E-cigarette or vaping product-associated lung injury: a review, Am J Med 133:657-663, 2020.