AUTHOR: B. Shoshana Zha, MD, PhD
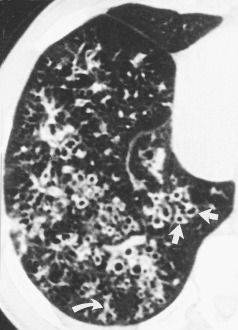
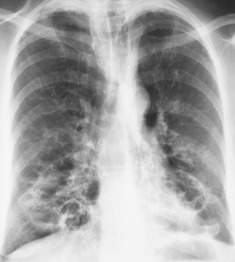
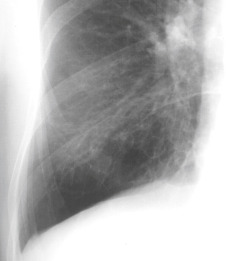
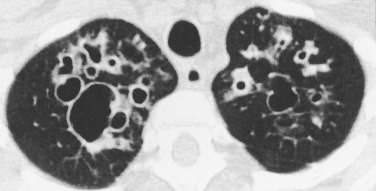
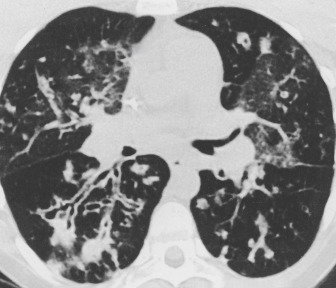
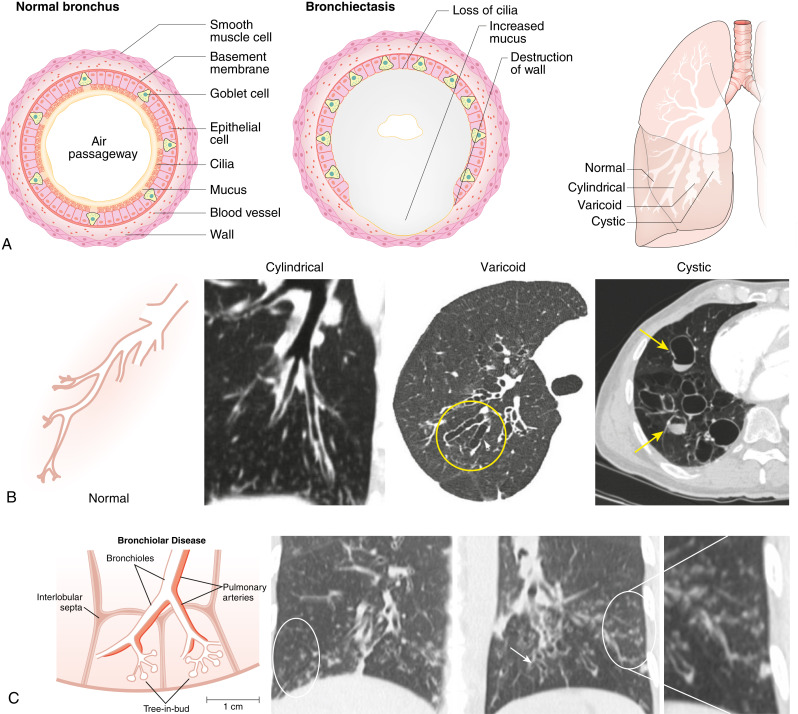
Bronchiectasis is a chronic lung disease resulting from dilatation of bronchi due to a variety of causes and propagated through an interplay of host factors, environment, and respiratory pathogens. Clinically, it is marked by a syndrome of cough, sputum production, and recurrent respiratory infections. It is confirmed radiographically by a lack of bronchial tapering and a bronchus-to-arterial ratio greater than 1.5. It can be divided into cylindric, varicose, and cystic subtypes, which often overlap and coexist, but may aid in diagnosis and overall prognosis.
| ||||||||||||||||||||
- The incidence and prevalence of bronchiectasis is steadily rising in the U.S. and Europe.1-4 Prevalence increases markedly with age, with peak incidence in those older than age 65. In the U.S., the average annual prevalence was approximately 700 per 100,000 persons among Medicare beneficiaries between 2012 and 2014,5 with likely higher prevalence given noted escalations of incidence.
- Bronchiectasis is more common in women than men.
- There are multiple identified etiologies that can vary by geographic region, yet idiopathic remains the most frequent diagnosis worldwide.6
- Table 1 summarizes conditions associated with bronchiectasis.
TABLE 1 Conditions Associated with Bronchiectasis
| Postinfectious Conditions | |||
| Childhood lower respiratory tract infections | |||
| Granulomatous infections | |||
| Necrotizing pneumonias in adults | |||
| Other respiratory infections | |||
| Primary Immune Disorders | |||
| Humoral defects | |||
| Cellular and/or mixed disorders | |||
| Neutrophil dysfunction | |||
| Other | |||
| Cystic Fibrosis (CF) | |||
| Classic CF | |||
| Variants of CF | |||
| Young syndrome | |||
| Alpha1-Antitrypsin Abnormalities | |||
| Deficiencies | |||
| Anomalies | |||
| Heritable Structural Abnormalities | |||
| Primary ciliary dyskinesia | |||
| Williams-Campbell syndrome | |||
| Mounier-Kuhn syndrome | |||
| Marfan syndrome | |||
| Sequestration, agenesis, hypoplasia | |||
| Idiopathic Inflammatory Disorders | |||
| Sarcoidosis | |||
| Rheumatoid arthritis | |||
| Ankylosing spondylitis | |||
| Systemic lupus erythematosus | |||
| Sjögren syndrome | |||
| Inflammatory bowel disease | |||
| Relapsing polychondritis | |||
| Inhalation and Obstruction | |||
| Gastroesophageal reflux/aspiration | |||
| Pneumonia | |||
| Toxic inhalation/thermal injury | |||
| Postobstruction accident | |||
| Foreign body | |||
| Tumors, benign and malignant | |||
| Extrinsic airway compression | |||
| Allergic bronchopulmonary aspergillosis/mycosis | |||
| Miscellaneous | |||
| Human immunodeficiency virus infection | |||
| Yellow nail syndrome | |||
| Radiation injury |
From Broaddus VC et al: Murray & Nadel’s textbook of respiratory medicine, ed 7, Philadelphia, 2022, Elsevier.
- Postinfectious (e.g., previous pneumonia, lung abscess, tuberculosis, nontuberculous mycobacterial infections, fungal infections, viral infections)
- Cystic fibrosis
- Ciliary dysfunction (primary ciliary dyskinesia, Kartagener syndrome)
- Chronic obstructive pulmonary diseases (e.g., chronic obstructive pulmonary disease [COPD], asthma)
- Impaired host defense (e.g., hypogammaglobulinemia, acquired immunodeficiency syndrome, chemotherapy)
- Localized airway obstruction (aspiration, congenital structural defects, foreign bodies, neoplasms)
- Inflammation (chronic gastroesophageal reflux disease [GERD], inflammatory pneumonitis, granulomatous lung disease, allergic aspergillosis)
- Congenital disorders such as tracheobronchomegaly (Mounier-Kuhn syndrome), cartilage deficiency (Williams-Campbell syndrome, Marfan syndrome), alpha-1 anti-trypsin deficiency
- Connective tissue and autoimmune diseases (Sjogren disease, ulcerative colitis, rheumatoid arthritis)
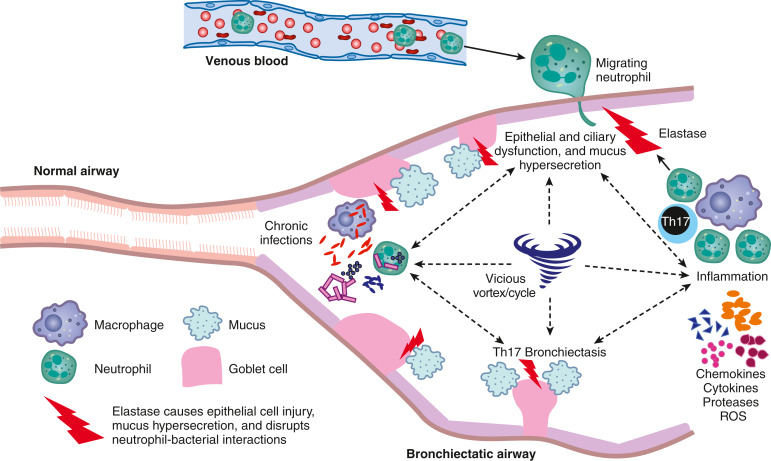
- Cellular pathophysiologic mechanism of bronchiectasis is illustrated in Fig. E1
Figure E1 Cellular pathophysiologic mechanism of bronchiectasis.
Cross-sectional view of the bronchiectatic airway demonstrating the components that play a role in the pathogenesis, including neutrophils, neutrophil elastase, goblet cells that produce mucus, and the chemokines and proinflammatory cytokines produced by macrophages and T helper type 17 cells (Th17). The epithelial and ciliary dysfunction, mucus hypersecretion, inflammation with unchecked protease (elastase) activity, chronic infection, and resultant bronchiectasis are intertwined and help perpetuate each other, creating the paradigm of the “vicious vortex/cycle” central to bronchiectasis pathogenesis. ROS, Reactive oxygen species.
From Broaddus VC et al: Murray & Nadel’s textbook of respiratory medicine, ed 7, Philadelphia, 2022, Elsevier.