AUTHOR: Glenn G. Fort, MD, MPH
Lyme disease is a multisystem inflammatory disorder caused by the transmission of a spirochete, Borrelia burgdorferi, via the bite of infected Ixodes ticks, taking 36 to 48 h for a tick to take a blood meal and transmit the infecting organism to the host. Table E1 summarizes the Centers for Disease Control and Prevention (CDC) Lyme disease surveillance case definition.
TABLE E1 2011 CDC Lyme Disease Surveillance Case Definition
| Definition of Erythema Migrans (EM) | |||
| A skin lesion that typically begins as a red macule or papule and expands over a period of days to weeks to form a large round lesion, often with partial central clearing. A single primary lesion must reach ≥5 cm in diameter. Secondary lesions also may occur. Annular erythematous lesions occurring within several hours of a tick bite represent hypersensitivity reactions and do not qualify as EM. The diagnosis of EM must be made by a physician. | |||
| Confirmed | |||
| Probable | |||
| Suspected | |||
|
CDC, Centers for Disease Control and Prevention; CSF, cerebrospinal fluid; Ig, immunoglobulin.
∗Exposure is defined as having been (≤30 days before onset of EM) in wooded, brushy, or grassy areas (i.e., potential tick habitats) in a county in which Lyme disease is endemic (in which at least two confirmed cases have been acquired or in which established populations of a known tick vector are infected with B. burgdorferi). A history of tick bite is not required.
† Positive culture for B. burgdorferi-or-two-tiered testing (positive or equivocal sensitive enzyme immunoassay [or immunofluorescent assay] followed by a Western immunoblot [both IgG and IgM tested if in the first 4 wk from symptom onset])-or-single-tier IgG immunoblot seropositivity.
‡ Recurrent, brief attacks of joint swelling in one or a few joints; lymphocytic meningitis, cranial neuritis (particularly facial palsy [may be bilateral]), radiculoneuropathy or, rarely, encephalomyelitis (requires CSF antibody production); acute onset of high-grade (second- or third-degree) atrioventricular conduction defects that resolve in days to weeks and are sometimes associated with myocarditis.
From 2011 CDC Lyme disease surveillance case definition. CDC, Centers for Disease Control and Prevention. Available at https://ndc.services.cdc.gov/case-definitions/lyme-disease-2011/.
Acrodermatitis chronica atrophicans
| ||||||||||||||||||||||||
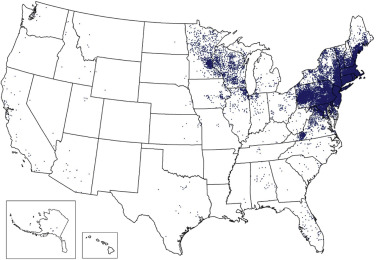
In the U.S., 4.4 cases/100,000 persons; it is the most common vector-borne infection in the U.S., with more than 30,000 new cases reported each yr. 90% of cases are found in Massachusetts, Connecticut, Rhode Island, New York, New Jersey, Pennsylvania, Minnesota, Wisconsin, and California. The area of transmission in the U.S. is expanding farther into the South and upper Northeast (Fig. E1). The disease also occurs in Europe and Asia with a different Ixodes tick vector. Table E2 summarizes principal vector ticks and spirochetes associated with Lyme borreliosis.
Figure E1 Reported cases of Lyme disease-United States, 2015.
Each dot represents one case of Lyme disease and is placed randomly in the patient’s county of residence. The presence of a dot in a state does not necessarily mean that Lyme disease was acquired in that state. The place of residence is sometimes different from the location where the patient became infected.
From Centers for Disease Control and Prevention: National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. In Cherry JD et al: Feigin and Cherry’s textbook of pediatric infectious diseases, ed 8, Philadelphia, 2019, Elsevier.
TABLE E2 Principal Vector Ticks and Spirochetes Associated With Lyme Borreliosis
| Tick Species | Distribution | Genotype of Borrelia burgdorferi |
|---|---|---|
| Ixodes scapularis | Eastern North America | B. burgdorferi sensu stricto |
| Ixodes pacificus | Western North America | B. burgdorferi sensu stricto |
| Ixodes ricinus | Western and Central Europe | B. garinii, B. afzelii, B. burgdorferi sensu stricto |
| Ixodes persulcatus | Central Europe and Asia | B. garinii, B. afzelii |
From Piesman J, Humair PF: The spirochetes and vector ticks of Lyme borreliosis in nature. In Sood SK (ed): Lyme borreliosis in Europe and North America, Hoboken, NJ, 2011, John Wiley & Sons.
Lyme disease may present in the following stages (Table E3):
- Early localized stage (incubation period 3 to 30 days): Early Lyme disease, erythema migrans (EM); skin rash, often at site of tick bite (the CDC has defined EM rash as an expanding red macule or papule that must reach at least 5 cm in size, with or without central clearing); target lesions from ECM can be found in 60% to 80% of localized infections; possible fever, myalgias 3 to 32 days after tick bite
- Early disseminated stage (incubation period 3 to 6 wk): Days to weeks later; multiorgan system involvement, including central nervous system (CNS) with aseptic meningitis-type picture or Bells palsy, joints (arthritis or arthralgias), cardiac including varying degrees of heart block; related to dissemination of spirochete
- Late stage (incubation period month to year): Month to year after tick exposure; affects central and peripheral nervous system, cardiac, joints
Common presenting signs and symptoms include:
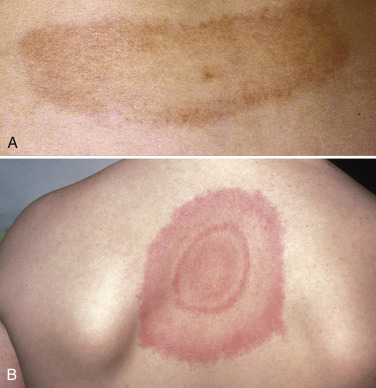
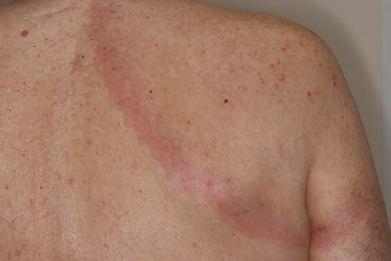
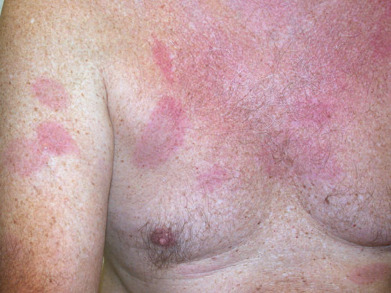
- EM (Fig. E2). Most patients with EM (about 80%) have a single lesion, but the bacteria can disseminate hematogenously to other sites in the skin and result in often smaller erythema migrans lesions (Figs. E3 and E4).
- Lymphadenopathy, neck pains, pharyngeal erythema, myalgias, hepatosplenomegaly.
- Patients will complain of malaise, fatigue, lethargy, headache, fever/chills, neck pain, myalgias, back pain.
(A) The site of the tick bite is visible near the center of the lesion. (B) Typical bull’s-eye lesion.
From Huppert HI, Dressler F: Lyme disease. In Cassidy J et al [eds]: Textbook of pediatric rheumatology, ed 6, Philadelphia, 2011, Saunders. In Hochberg MC: Rheumatology, ed 7, Philadelphia, 2019, Elsevier.
Figure E3 Lyme disease (erythema chronicum migrans).
From Micheletti RG, et al: Andrews’ diseases of the skin, clinical atlas, ed 2, Philadelphia, 2023, Elsevier.
Figure E4 Disseminated Lyme disease (multiple erythema migrans lesions).
From Micheletti RG, et al: Andrews’ diseases of the skin, clinical atlas, ed 2, Philadelphia, 2023, Elsevier.
TABLE E3 Clinical Manifestations of Lyme Disease
| Early Localized Infection | |||
| Early Disseminated Infection | |||
| Late Disease | |||
EM, Erythema migrans.
From Firestein GS, et al: Firestein & Kelley’s textbook of rheumatology, ed 11, Philadelphia, 2021, Elsevier.