AUTHOR: Glenn G. Fort, MD, MPH

DefinitionYellow fever is a mosquito-borne infection, primarily of the liver, with systemic manifestations caused by the yellow fever virus (YFV), a flavivirus that infects the liver. The clinical spectrum ranges from asymptomatic infection to life-threatening disease with severity and mortality highest in the elderly.
SynonymTropical hemorrhagic fever from YFV
| ICD-10CM CODE | | A95.9 | Yellow fever, unspecified |
|
Epidemiology & DemographicsGeographic Distribution
- South America and Africa, in countries between +15 and -15 degrees latitude (Fig. E1)
- The World Health Organization estimates there are more than 200,000 cases/yr with 30,000 deaths/yr. >90% of cases occur in Africa
- Outbreaks of yellow fever occurred in 2015 to 2018 in Angola and starting in 2016 in Brazil, which led that nation to recommend vaccination for the entire country instead of provinces that had traditionally had cases. In 2021, nine west African countries reported cases
Figure E1 Geographic distribution of yellow fever in Africa and the Americas.
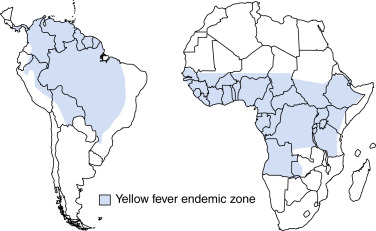
From Cherry JD et al: Feigin and Cherry’s textbook of pediatric infectious diseases, ed 8, Philadelphia, 2019, Elsevier.
IncidenceApproximate attack rates of 3% in Africa and Amazon
PrevalenceEndemic areas: 20% of population
Predominant SexIn Africa and the Amazon, male agricultural workers
Physical Findings & Clinical Presentation
- Most patients with yellow fever are asymptomatic.
- The onset of illness appears suddenly 3 to 6 days after the bite of an infected mosquito.
- Viremic (early) phase:
- Fever, chills
- Severe headache
- Lumbosacral pain
- Myalgias, nausea, malaise
- Conjunctivitis
- Relative bradycardia (Faget sign)
- After brief recovery, toxic phase:
- Jaundice
- Oliguria
- Albuminuria
- Hemorrhage
- Encephalopathy
- Shock
- Acidosis
- Case-fatality rate: 25% to 50%.
Etiology
- YFV (L. flavus): Small enveloped single-strand RNA virus
- Flavivirus infects hepatic cells
- Late in infection, cytopathic effects (antibody- and cell-mediated) produce pathology
- Vector
- Aedes aegypti (urban)
- Aedes spp., Haemagogus (especially in Amazon) mosquitos (sylvan)
- Primary hosts: Humans and simian species
- Exists in two transmission cycles:
- Sylvatic or jungle cycle involving mosquitoes and nonhuman primates
- Urban cycle involving mosquitoes and humans
Pathogenesis & Pathology
- Virus replication begins at site of mosquito bite, spreading to lymphatic channels and regional lymph nodes. Viremic spread to other organs, especially liver, spleen, and bone marrow.
- Shock and fatal illness result from direct damage to organs and vasoactive cytokines.
- Viral antigen found in hepatocytes, kidneys, and myocardium.
- Midzone necrosis of liver lobules primarily affected and is highly characteristic.
- Hemorrhages of mucosal surfaces of GI tract.
