AUTHORS: Fahad Ali, MDand Rory Merritt, MD, MEHP
Acetaminophen (APAP) poisoning is a disorder caused by excessive intake of APAP and is manifested by jaundice, nausea, vomiting, abdominal discomfort, and potential death from hepatic necrosis if not treated appropriately.
| ||||||||||||
- APAP is one of the most widely prescribed antipyretics and analgesics in the U.S. Potentially toxic ingestions, both intentional and unintentional, exceed 100,000 cases annually in the U.S.
- APAP is the most commonly potential toxic pharmaceutical reported to U.S. Poison Control Centers.
- APAP is available in more than 100 over-the-counter combination formulations, such as DayQuil/NyQuil Cold and Flu, Excedrin, and Robitussin Cold and Flu.
- APAP toxicity is the number one cause of liver transplant in the U.S. Death rate is approximately 1 in 1000 persons. Nearly 50% of exposures occur in children ≤6 yr.
- Hepatic necrosis is most likely to occur in people who (1) are chronically malnourished, (2) have alcohol use disorder, (3) have chronic liver disease, and (4) use other potentially hepatotoxic medications.
- Combination opioid and acetaminophen medications are an important source of APAP toxicity.
- The physical examination may vary depending on the amount of time since ingestion.
- Phase I (0-24 hr): Initial symptoms may be mild or absent and may consist of anorexia, diaphoresis, malaise, nausea, vomiting, lethargy, and a subclinical rise in transaminase levels. In a massive APAP ingestion, altered mental status and lactic acidosis can occur in phase I.
- Phase II (24-72 hr): Right upper quadrant pain, vomiting, somnolence, tachycardia, jaundice, hypotension, and continued increase in transaminases.
- Phase III (72-96 hr): Hepatic necrosis with abdominal pain, jaundice, hepatic encephalopathy, coagulopathy, hypoglycemia, renal failure, fatality from multiorgan failure.
- Phase IV (4 days to 2 wk): Complete resolution of symptoms and resolution of organ failure.
- Table E1 summarizes the four phases/stages of acetaminophen poisoning.
TABLE E1 Four Stages of Acetaminophen Poisoning
From Adams J (ed), Barton E et al (associate eds): Emergency medicine: clinical essentials, ed 2, Philadelphia, 2013, Saunders.
|
ALT, Alanine transaminase; AST, aspartate transaminase; INR, international normalized ratio.
From Adams J (ed), Barton E et al (associate eds): Emergency medicine: clinical essentials, ed 2, Philadelphia, 2013, Saunders.
- The amount of APAP necessary for hepatic toxicity varies with the patient’s body size and hepatic function. APAP hepatotoxicity occurs when its typical metabolism pathway is overwhelmed, resulting in glutathione depletion and accumulation of the toxic metabolite, NAPQI.
- When prescribing APAP, it is recommended that APAP intake should not exceed 3 to 4 g for adults and 90 mg/kg in children within a 24-hr period. A potentially toxic single ingestion of APAP is 7.5 g for adults or 150 mg/kg in a child.
- There is no standard accepted definition, but a massive APAP ingestion is characterized by a single ingestion of >30 g to >50 g or an APAP concentration of greater than 250 mcg/ml to 300 mcg/ml in 4 h.
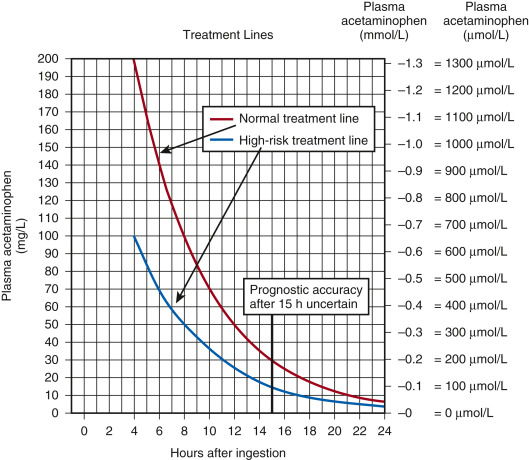
- A standardized nomogram (Fig. 1 ) is used to determine potential hepatic toxicity by knowing the APAP plasma level and the number of hr after ingestion. See the APAP ingestion algorithm.
The High-Risk Line Has Been Adopted as the Standard and Only Treatment Line in the United Kingdom. This is Done to Avoid Confusion and to Reduce the Risk of Undertreatment in Patients with Chronically Induced Hepatic Enzymes, Such as Those Receiving Antiepileptic Medication, Chronic Ethanol Abusers, or Smokers. If There is any Doubt About the Number of Hours Since Ingestion, or if There is any Suggestion of a Staggered Overdose or Chronic Overingestion, NAC Should Be Administered.
From Paracetamol overdose: new guidance on treatment with intravenous acetylcysteine, Drug Safety Update 6[2]:A1, 2012.