AUTHORS: Frank Sanchez, MD, MBA, and Glenn G. Fort, MD, MPH
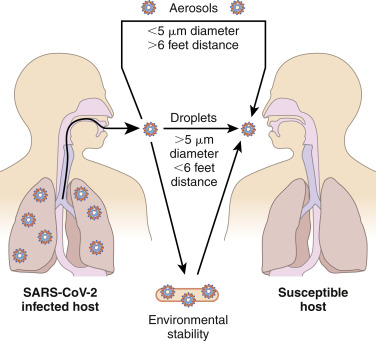
COVID-19 disease is a human respiratory illness, transmitted person-to-person primarily via respiratory droplets (Fig. 1) as well as contact with contaminated surfaces, caused by a novel coronavirus, SARS-CoV-2, that emerged in December 2019 in Wuhan, China. By March 2020 it was declared a worldwide pandemic by the World Health Organization. Over time, like many viruses, mutations occurred that significantly changed the clinical course of the disease. These variants are labeled by distinct phylogenetic classification systems or by the Greek alphabet as per the WHO: Alpha, Beta, Gamma, Delta, and Omicron, etc.
The major mode of transmission is by droplets (>5 μm diameter), generated by coughing, sneezing, or talking, consistent with the known presence of virus in the upper respiratory tract. Aerosol transmission has been strongly suggested by certain spreading events, in which small particles (<5 μm) can spread more than 6 feet and remain suspended. Fomite transmission, by touching surfaces and spreading virus to the face, happens but appears to be a minor mode of transmission.
From Broaddus VC et al: Murray & Nadel’s textbook of respiratory medicine, ed 7, Philadelphia, 2022, Elsevier.
| ||||||||||||||||||||
After originating in China, the worst countries/areas affected worldwide include the United States, Europe, Brazil, India, and Russia. Most current information on incidence: www.coronavirus.jhu.edu.
The pandemic started in the winter of 2019. With the advent of vaccines directed toward COVID-19, the incidence has steadily decreased.
At the time this chapter was written, the COVID-19 pandemic has claimed an estimated 15 million lives, including more than 1 million in the U.S. alone.1
Men are more at risk for worse outcomes and death, independent of age, with COVID-19. Whereas males and females have the same prevalence of COVID-19, male patients have a higher mortality rate. Children also get infected but have milder disease, much better outcomes, and a lower mortality rate of about 0.1% in the United States.
- Overall, where sex or gender data are available, it appears that females are more often affected, but disease is more severe in males.
Other Risk Factors/Associations:
Various underlying medical conditions have been associated with increased risk for severe disease, especially if they are not well controlled:
- Chronic kidney disease
- Chronic obstructive pulmonary disease
- Immunosuppression because of previous solid organ transplant
- Malignancy
- Obesity (body mass index [BMI] of 30 or higher)
- Serious cardiac conditions (e.g., heart failure, coronary artery disease, cardiomyopathy)
- Sickle cell disease
- Diabetes type 2
Conditions That May Be Associated With Higher Risk for Severe Disease:
- Asthma (moderate to severe)
- Cerebrovascular disease
- Cystic fibrosis
- Hypertension
- Immunodeficiency from various other causes (e.g., bone marrow or hematopoietic stem cell transplant, primary immunodeficiencies, HIV disease, chronic treatment with corticosteroids or other agents with immunosuppressive effects)
- Neurologic dysfunction
- Chronic liver disease
- Pregnancy
- Pulmonary fibrosis
- Smoking
- Thalassemia
- Diabetes type 1
- Children with medically complex conditions (e.g., neurologic, metabolic, genetic, cardiac) are also at higher risk for severe disease.
- Residents of nursing homes and long-term care facilities are at high risk for acquiring infection and for severe disease, probably owing to a combination of heightened transmission in a close-quarters community and prevalence of compromised health status.
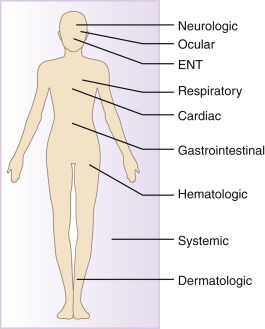
Figure E1 Clinical manifestations of COVID-19.
Neurologic manifestations are present in 36% to 57% of patients, with the most common findings of dizziness, headache, and impaired consciousness. The most common ocular manifestation is conjunctivitis. Disorders of taste and smell are very common ear, nose, and throat (ENT) manifestations, affecting as many as 89% of patients. Cough (45% to 80%) and dyspnea (20% to 55%) are common respiratory manifestations. Arrhythmias affect 7% to 17% of patients, and myocarditis is a cardiac manifestation described in several case reports. Gastrointestinal manifestations are less common (7% to 9% in metaanalyses), but they may be the only symptoms in some patients. Elevated D-dimer levels are a common hematologic disorder and likely confer an increased risk for thromboembolism. Rash may present as a dermatologic feature in up to 20% of patients with COVID-19, and these rashes may appear as erythema, urticaria, or vesicles. Fever, myalgias, and fatigue are common systematic symptoms in the influenza-like illness characteristic of COVID-19.
From Broaddus VC et al: Murray & Nadel’s textbook of respiratory medicine, ed 7, Philadelphia, 2022, Elsevier.
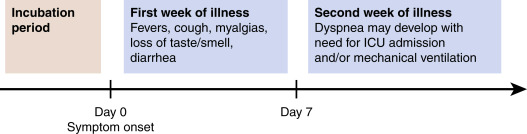
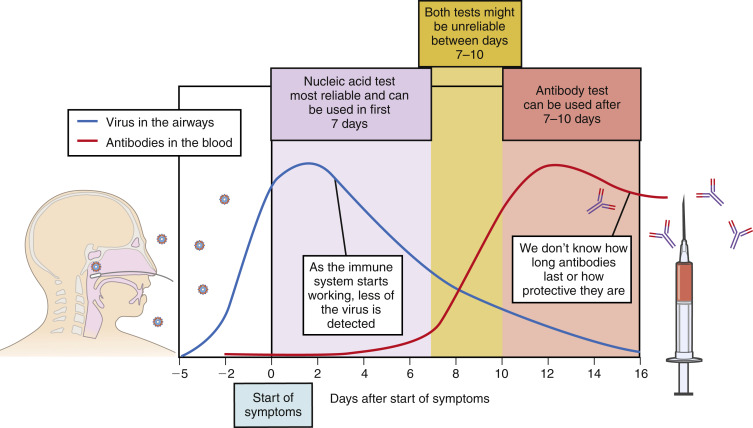
- Incubation period: Within 14 days of exposure but most patients exhibit symptoms by day 4 to 5 after exposure (Fig. 3)
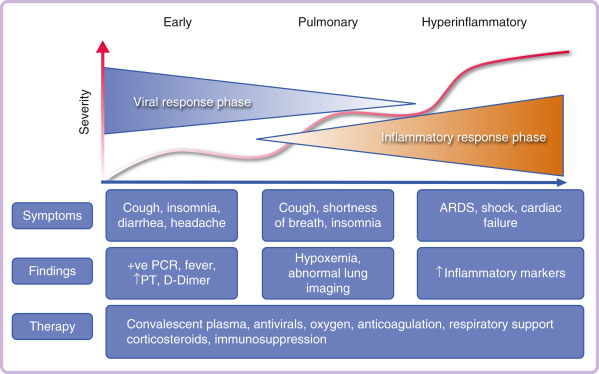
- Spectrum of disease (Fig. 4):
- Asymptomatic disease: May be as high as 30% to 40%
- Mild disease: No or mild pneumonia-about 80% of symptomatic cases
- Severe disease: Dyspnea, hypoxia, or >50% lung involvement within 24 to 48 hours-less than 10% with new variants
- Critical disease: Respiratory failure, shock, or multiorgan failure-less than 5% with new variants
- Overall case fatality rate is less than 2%
- Initial presentation: Pneumonia manifested by fever, cough, dyspnea, and bilateral infiltrates on chest imaging is the most serious presentation of the infection; however, in a study of 370,00 confirmed cases in the United States, a variety of symptoms were associated with COVID-19 infection:
- Available data suggest that as least one third of SARS-CoV-2 infections are asymptomatic.2
- In some patients, gastrointestinal manifestations (nausea and diarrhea) may be how the infection presents.
- Clinicians should be particularly attuned to pulmonary and hemodynamic indicators of severe disease:
- Patients with severe disease may appear quite ill, with tachypnea and labored respirations.
- Patients in apparent distress require immediate assessment of airway, breathing, and circulation (e.g., pulses, blood pressure, O2Sat).
- Clinicians should be aware of the COVID-19-related phenomenon of silent (or “happy”) hypoxemia; absence of signs of respiratory distress may be misleading.
- Oxygenation should be assessed promptly by peripheral saturation (e.g., pulse oximetry).
- Fever is typical, occasionally exceeding 39° C. Patients in the extremes of age or with immunodeficiency may not develop fever.
- Conjunctival secretions, injection, and chemosis have been reported.
- A variety of skin changes have been described, including erythematous rashes, purpura, petechiae, and vesicles; Pernio (chilblain)-like acral lesions (COVID Toes) or Janeway lesions have been seen, particularly in young patients.
- Hypotension, tachycardia, and cool/clammy extremities suggest shock.
- In children, hypotension plus two or more of the following criteria may be indicative of COVID-19-associated multisystem inflammatory syndrome:
- Altered mental status
- Tachycardia (heart rate more than 160 beats per minute in infants or 150 in older children) or bradycardia (heart rate less than 90 in infants or 70 in older children)
- Prolonged capillary refill (more than 2 seconds) or warm vasodilation and bounding pulses
- Tachypnea
- Mottled skin, petechiae, or purpura
- Oliguria
- Hyperthermia or hypothermia
Figure 4 The phases of COVID-19 infection.
Many patients are infected but are totally asymptomatic. In some patients, the disease progresses in a variable course through several overlapping phases, with changes in symptoms, laboratory findings, and therapy requirements.
From Kryger M et al: Principles and practice of sleep medicine, ed 7, Philadelphia, 2023, Elsevier.
Figure 3 Clinical course of COVID-19.
The presymptomatic incubation period may last a median of 5 days. During the first week of illness, symptoms tend to be flu-like, with the added symptoms of losses of taste and smell. Severe disease may develop in the second week, with dyspnea and the need for intensive care unit (ICU) treatment. Many patients remain asymptomatic during their illness.
From Broaddus VC et al: Murray & Nadel’s textbook of respiratory medicine, ed 7, Philadelphia, 2022, Elsevier.
- Respiratory failure:
- High-flow nasal cannula (HFNC) is recommended over noninvasive positive pressure ventilation (NIPPV).
- If hypoxemia persists and intubation is not indicated, it is recommended to use a trial of awake prone positioning to improve oxygenation.
- If patient requires intubation, use a higher positive end expiratory pressure (PEEP) strategy over a lower PEEP strategy.
- If still hypoxemic despite intubation, prone ventilation for 12 to 16 hours a day is recommended.
- Cardiac and cardiovascular complications:
- Acute coronary syndrome (ACS): There has been an increased coronary artery thrombus burden in patients with COVID-19 and an increased risk of developing a STEMI. This is likely related to the hypercoagulable state produced by COVID-19. The ability to reperfuse the patient with PCI or fibrinolysis will depend on how ill the patient is.
- Myocarditis is a known complication and likely results from a combination of direct viral injury and cardiac damage due to the host’s immune response. Clinical findings include changes in electrocardiogram or cardiac biomarkers and impaired cardiac function. Cardiac magnetic resonance imaging or cardiac computed tomography (CT) with angiography may help in the diagnosis.
- Arrhythmias, congestive heart failure, and cardiac shock can also occur.
- Thromboembolic complications: Severe COVID-19 illness is associated with intense inflammation, leading to high rates of thrombotic complications that lead to higher morbidity and mortality rates. One study showed a 25% incidence of deep vein thrombosis (DVT) in severe COVID-19, and another study found a combined incidence of 31% for DVT, pulmonary embolus, and arterial embolism.
- Histopathologic studies show diffuse alveolar damage with profound inflammation, thrombosis, and thrombotic microangiopathy of small vessels and capillaries of the lung.
- Measurement of D-dimer, fibrinogen, prothrombin time, INR, and APTT should be obtained every 48 hours.
- Prophylaxis against DVT is recommended for all patients with COVID-19 in the hospital using low-molecular-weight heparin, unfractionated heparin for those in renal failure, or fondaparinux for those with heparin-induced thrombocytopenia, as long as platelet count is above 25 × 109/L.
- Patients with a D-dimer level of 3.0 μg/ml or higher should undergo screening with point-of-care ultrasound to rule out DVT and receive more intensive prophylaxis.
- Other complications: Stroke
- The SARS-CoV-2 is a coronavirus, an enveloped positive stranded RNA virus. It is in the same subgenus as the severe acute respiratory virus (SARS) as well as several bat coronaviruses. It is not known if there was direct transmission from bats or some other intermediary host.
- The name coronavirus comes from the Latin word meaning “crown” or “halo” because on two-dimensional electron microscopy the virus has a “crown-like” appearance caused by club-shaped spike peplomers covering the surface.
- The virus enters cells through an endocytosis pathway, using surface spike (S) proteins to bind to angiotensin-converting enzyme 2 (ACE-2) and dipeptidyl peptidase 4 (DPP4) receptors on the ciliated bronchial epithelial cells and type II pneumocytes. Once the virus enters a host cell, viral RNA is exposed and replication occurs.
- COVID-19 Variants
- Alpha Variant (B.1.1.7 lineage): First seen in the UK in December 2020 and felt to be 50% to 75% more transmissible than previous circulating strain.
- Beta Variant (B.1.351 lineage): Identified in South Africa in late 2020.
- Delta (B.1.617.2 lineage): First identified in India in December 2020 and had become the most prevalent variant in India, United Kingdom, and the U.S. by summer of 2021. It is felt to be much more transmissible than previous variants, and it appears current vaccines are less efficacious against this variant.
- Omicron: First identified in Africa in fall 2021, became most prevalent strain by winter 2021-2022. A much higher transmission rate than prior strains but with milder symptoms.
- The CDC now houses a collection of Variants of Concern (VOC) and Variants of Interest (VOI) on their website to keep track of emerging variants that may lead to another pandemic and can be found at: https://www.cdc.gov/coronavirus/2019-ncov/variants/.