AUTHOR: Fred F. Ferri, MD
Prostate cancer is a neoplasm involving the prostate. Various classifications have been developed to evaluate malignancy potential and prognosis.
- The degree of malignancy varies with the stage:
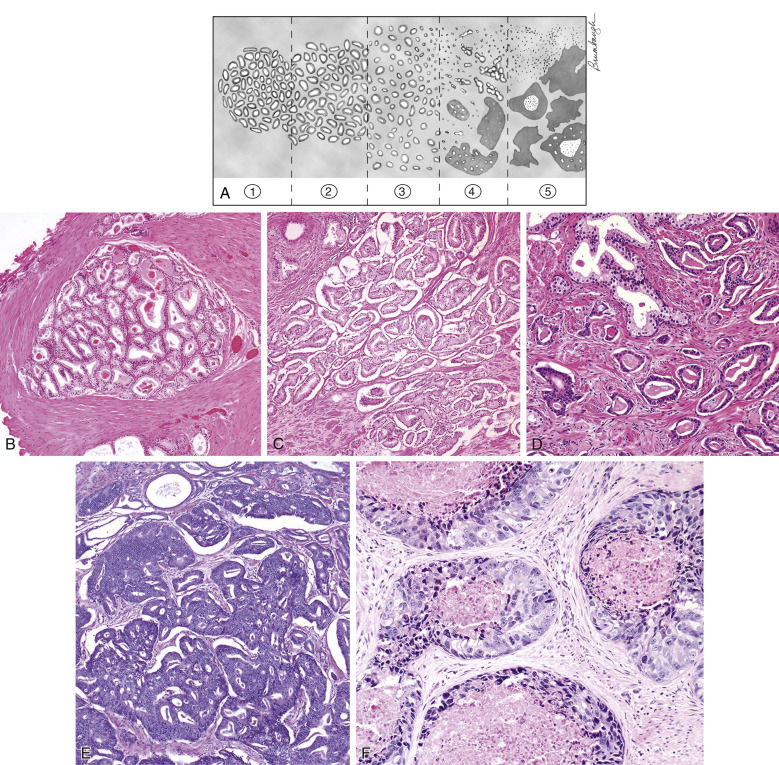
- In the Gleason classification (Box 1 and Fig. E1), two histologic patterns are independently assigned numbers 1 to 5 (best to least differentiated). These numbers are added to give a total tumor score between 2 and 10. Prognosis is best for highly differentiated tumors (e.g., Gleason score 2-6) compared with most poorly differentiated tumors (Gleason score 7-10).
- Another commonly used classification is the Tumor-Node-Metastasis (TNM) classification of prostate cancer (Table 1).
- Table 2 summarizes the definition of risk groups and biopsy criteria.
TABLE 2 Definition of Risk Groups
| Risk Group | Clinical Stage | PSA (ng/ml) | Gleason Score | Biopsy Criteria |
|---|---|---|---|---|
| Low | T1a or T1c | <10 | 2-6 | Unilateral or <50% of core involved |
| Intermediate | T1b, T1c, or T2a | <10 | 3 + 4 = 7 | Bilateral |
| High | T1b, T1c, T2b, or T3 | 10-20 | 4 + 3 = 7 | >50% of core involved or perineural invasion or ductal differentiation |
| Very high | T4 | >20 | 8-10 | Lymphovascular invasion or neuroendocrine differentiation |
From Wein AJ et al: Campbell-Walsh urology, ed 11, Philadelphia, 2016, Elsevier.
| T Stage | ||
| Tx | Primary tumor is not assessable | |
| T0 | There is no evidence of primary tumor | |
| T1 | Tumor is not clinically palpable or detected with imaging | |
| T1a | An incidental histologic finding in ≤5% of resected tissue (e.g., TURP) | |
| T1b | An incidental histologic finding in >5% of resected tissue (e.g., TURP) | |
| T1c | Tumor is identified by needle biopsy | |
| T2 | Prostate-confined tumor that is clinically palpable or detected with imaging | |
| T2a | Tumor involves ≤1/2 of one prostate lobe | |
| T2b | Tumor involves >1/2 of one prostate lobe (but not both lobes) | |
| T2c | Tumor involves both lobes | |
| T3 | There is tumor extension through the prostate capsule | |
| T3a | Unilateral or bilateral tumor extension through the prostate capsule | |
| T3b | Seminal vesical involvement | |
| T4 | Tumor invades structures other than the seminal vesicles (e.g., the bladder neck, rectum, or pelvic wall) | |
| N Stage | ||
| Nx | The lymph nodes are not assessable | |
| N0 | There is no tumor spread | |
| N1 | There is tumor spread to one or more regional pelvic nodes | |
| M Stage | ||
| M0 | There is no tumor spread beyond the regional pelvic nodes | |
| M1 | There is tumor spread beyond the regional pelvic nodes | |
| M1a | Tumor spread to nodes outside of the pelvis | |
| M1b | Tumor spread to bones | |
| M1c | Tumor spread to other organs (e.g., lung, liver and brain) ± bone involvement | |
From Grant LA: Grainger & Allison’s diagnostic radiology essentials, ed 2, Philadelphia, 2019, Elsevier.
BOX 1 2005 International Society of Urological Pathology Modified Gleason System
From Wein AJ et al: Campbell-Walsh urology, ed 11, Philadelphia, 2016, Elsevier.
| ||||||||||||
Figure E1 The Gleason grading system.
(A) Schematic diagram of the Gleason grading system. (B) Gleason pattern 1: Well-circumscribed nodule of closely packed glands. (C) Gleason pattern 2: Nodule with more loosely arranged glands. (D) Gleason pattern 3: Small glands with an infiltrative pattern between benign glands. (E) Gleason pattern 4: Large irregular cribriform glands. (F) Gleason pattern 5: Solid nests of tumor with central comedonecrosis.
From Wein AJ et al: Campbell-Walsh urology, ed 11, Philadelphia, 2016, Elsevier.
- Prostate cancer has surpassed lung cancer as the most common nonskin cancer in men.
- In the United States, more than 220,000 new cases are diagnosed yearly, and nearly 30,000 males die from prostate cancer each year (second leading cause of death from cancer in U.S. men).
- Incidence of prostate cancer increases with age: Uncommon <50 yr; 80% of new cases are diagnosed in patients aged ≥65 yr. Widespread prostate-specific antigen (PSA) testing has doubled the incidence of prostate cancer and the lifetime risk for prostate cancer to approximately 16%. Prostate cancer is also diagnosed earlier, and the incidence of clinically “silent” T1 tumors has increased from 17% in 1989 to 48% in 2001 since the advent of PSA screening. Currently, approximately 80% of prostate cancer cases are diagnosed as localized disease and only 4% as metastatic disease. The incidence of metastatic prostate cancer for middle-aged men was stable from 2004 to 2010 and then increased from 12 to 17 cases/100,000 from 2010 to 2018.1 It is unclear if this increase was due to U.S. Preventive Services Task Force (USPSTF) recommendations against PSA screening in 2008 and 2012 or to more aggressive diagnostic strategies.
- Prostate cancer is found at autopsy in more than half of U.S. men older than 50 yr but is the cause of death in only 3%.
- Average age at time of diagnosis is 72 yr.
- Blacks in the U.S. have the highest incidence of prostate cancer in the world (one in every nine males).
- Incidence is low in Asians.
- Approximately 9% of all prostate cancers may be familial. Obesity is a risk factor for prostate cancer. High-fat, low-fiber diet increases risk. High insulin levels may also increase the risk of prostate cancer. Dietary supplementation with vitamin E has been reported to significantly increase the risk of prostate cancer among healthy men. Linkage studies have implicated chromosome 17p21-22 as a possible location of a prostate-cancer susceptibility gene. Germline mutations in HOXB13 are associated with a significantly increased risk of hereditary prostate cancer.
- Mortality rates of prostate cancer have declined substantially in the past 15 yr from 34% in 1990 to <20% currently.
- Generally silent disease until it reaches advanced stages.
- Bone pain and pathologic fractures may be initial symptoms of prostate cancer.
- Local growth can cause symptoms of outflow obstruction.
- Digital rectal examination (DRE) may reveal an area of increased firmness; 10% of patients will have a negative DRE.
- Prostate may be hard, fixed, with extension of tumor to the seminal vesicles in advanced stages.