Author: Glenn G. Fort, MD, MPH
Rabies is a fatal zoonotic illness caused by a number of species of neurotropic viruses in the Rhabdoviridae family and transmitted to human beings by the bite of an infected animal.
Approximately one to three U.S. cases annually; 25 human cases of rabies in the U.S. from 2009 to 2018. Of these, seven cases were imported rabies in people who were exposed to rabid animals in endemic nations and one to two cases were in tissue or organ transplantation recipients. Of the 25 cases, 23 died and two survived. In 2021 there were five cases of rabies in the U.S. from bats. Tens of thousands of cases of rabies occur worldwide annually.
In the U.S., most cases are transmitted by bats; in the world, most cases are transmitted by dogs.
- Incubation period is usually 1 to 3 mo in humans but can range from several days to 8 yr
- Shorter incubation period with bites on the face. Longer if extremities involved
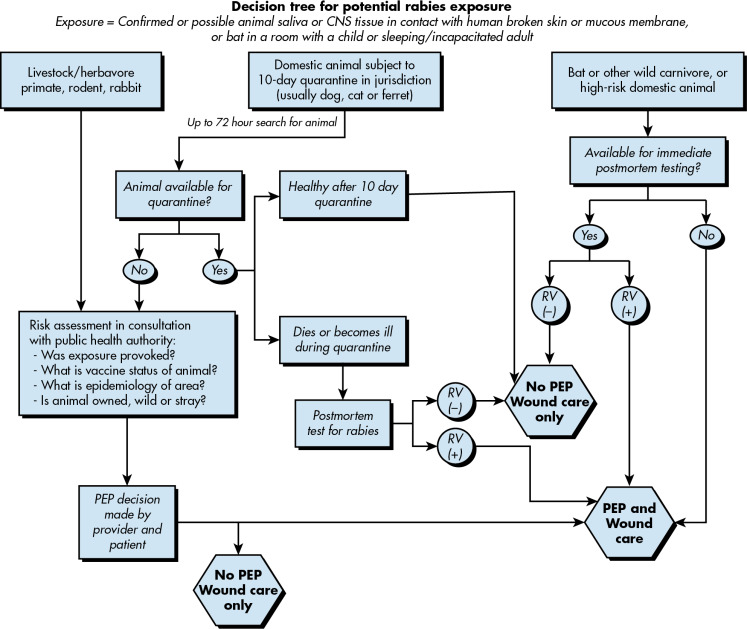
- In domestic animals, a quarantine of 10 days is usually mandated
- Prodrome:
- Acute neurologic period, with objective evidence of central nervous system involvement
- Two types of presentation
- "Furious" (encephalopathic) rabies (80% of cases):
- "Dumb" (paralytic) rabies (20% of cases):
- Rabies virus: Bullet-shaped viruses that belong to Rhabdoviridae family, genus Lyssavirus
- Cases in the U.S. are associated with:
- Small rodents (squirrels, mice, rats, chipmunks) and lagomorphs such as rabbits almost never have rabies and have never been a source of transmission of rabies to humans in the U.S.
- In 13/25 cases of rabies occurring in the U.S. from 2009 to 2018, exposure to bats was thought to be the source, although many did not have a clinically evident bite or scratch
- Imported cases are usually associated with dogs
- Unusual acquisition: