Author: Klodia M. Hermez, DO and Snigdha T. Reddy, MD
Chronic kidney disease (CKD) is diagnosed when there is evidence of kidney damage for more than 3 mo (urine albumin >30 mg/g creatinine, hematuria, or parenchymal abnormalities) and/or decreased kidney function (glomerular filtration rate [GFR] <60 ml/min per 1.73 m2.1 Advanced CKD, when GFR is <30 ml/min per 1.73 m2, is characterized by accumulation of metabolic waste products in the blood, electrolyte abnormalities, mineral and bone disorders, and anemia. The pathophysiology of CKD and its manifestations are summarized in Table 1.
TABLE 1 Pathophysiology of Chronic Kidney Disease
| Manifestation | Mechanisms | ||
|---|---|---|---|
| Accumulation of nitrogenous waste products | Decrease in glomerular filtration rate | ||
| Acidosis | |||
| Sodium retention | |||
| Sodium wasting | |||
| Urinary concentrating defect | |||
| Hyperkalemia | |||
| Renal osteodystrophy |
| ||
| Growth retardation | |||
| Anemia | |||
| Bleeding tendency | Defective platelet function | ||
| Infection | |||
| Neurologic symptoms (fatigue, poor concentration, headache, drowsiness, memory loss, seizures, peripheral neuropathy) | |||
| Gastrointestinal symptoms (feeding intolerance, abdominal pain) | |||
| Hypertension | |||
| Hyperlipidemia | Decreased plasma lipoprotein lipase activity | ||
| Pericarditis, cardiomyopathy | |||
| Glucose intolerance | Tissue insulin resistance |
From Kliegman RM: Nelson textbook of pediatrics, ed 21, Philadelphia, 2020, Elsevier.
The 2012 Kidney Disease: International Global Outcomes classifies CKD with a C-G-A format: Cause (etiology), GFR (G1 to G5), and albuminuria (A1 to A3) by urine albumin-to-creatinine ratio. This format is recommended when documenting and discussing CKD. Fig. E1 depicts how CKD prognosis worsens with either increasing levels of albuminuria or declining GFR. Until now, most physicians used the 2009 CKD EPI (chronic kidney disease epidemiology collaboration) to calculate estimated glomerular filtration rate (eGFR), which took into account race. Recently in 2021, the joint NKF-ASN task force recommended the use of the new 2021 CKD EPI equation that excludes race from this calculation.
Figure E1 Depiction of chronic kidney disease (CKD) classification: G stages and A stages.
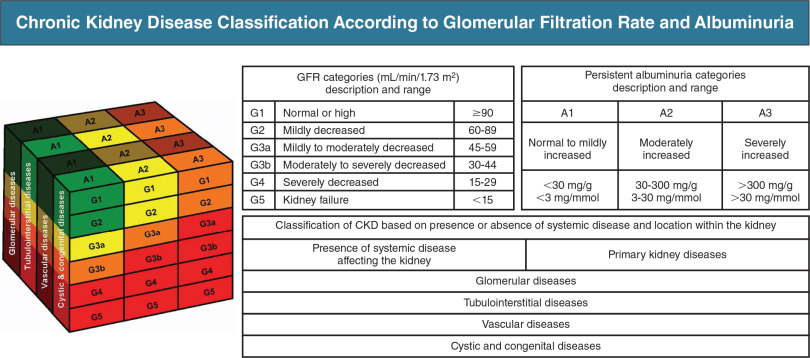
The cube denotes how the three components of the CKD classification scheme (glomerular filtration rate [G], albuminuria [A], and underlying kidney disease) interact to influence the risk of progression to kidney failure. This risk is represented in qualitative terms (lowest to highest) by green, yellow, orange, and red colors.
(From Floege J et al: Comprehensive clinical nephrology, ed 7, Philadelphia, 2024, Elsevier.)
| ||||||||||||||||||||||||||||||||||||||||
- In the U.S., 37 million adults are estimated to have CKD, and approximately 90% do not know that they have diminished kidney function.2 One in three U.S. adults is at risk for CKD.2,3
- Total Medicare spending on CKD and end-stage renal disease (ESRD) patients exceeds $120 billion USD. Accompanying chronic diseases such as diabetes and heart failure compound the cost of caring for these individuals, and CKD is considered a "cost multiplier," accounting for 25% of Medicare expenditures. Spending per patient-yr for patients with all three chronic conditions of CKD, diabetes, and heart failure was more than twice as high ($40,516) as for patients with only CKD.3
- CKD will be the fifth highest cause of years of life lost world-wide by 2040.4
- Kidney transplantation represents the best option for kidney replacement therapy, offering greater longevity and quality of life while having the lowest overall cost. Mortality is significantly lower (48.9 per 1000) versus those on dialysis (160.8 per 1000).2
- Most patients with early CKD have no or few symptoms on physical exam
- Skin pallor, ecchymosis
- Sleep disorder
- Hypertension, edema, jugular venous distention
- Leg cramps, restless legs, peripheral neuropathy
- Emotional lability, depression, decreased cognitive function
- Uremic frost, odor, and fetor in severe cases
- Clinical presentation varies with the degree of kidney disease and its underlying etiology. Common symptoms are generalized fatigue, nausea, anorexia, pruritus, sleep disturbance, smell and taste disturbances, hiccoughs, and seizures
- Diabetes mellitus
- Hypertension
- Glomerular disease
- Failed kidney transplant
- Interstitial nephritis (e.g., drug hypersensitivity, analgesic nephropathy)
- Obstructive nephropathies (e.g., nephrolithiasis, prostatic disease)
- Congenital disease (e.g., Alport syndrome, polycystic kidney disease)
- Vascular diseases (renal artery stenosis, hypertensive nephrosclerosis)
- Autoimmune disorders
- Acute kidney injury (AKI)