Author: Eric Ebert, MD and Rory Merritt, MD, MEHP
Acetaminophen (APAP) poisoning is caused by excessive intake of APAP and is characterized by jaundice, nausea, vomiting, abdominal discomfort, and potential death from hepatic necrosis if not treated appropriately.
| ||||||||||||
- APAP is the most widely used antipyretic and analgesic in the U.S. Potentially toxic ingestions, both intentional and unintentional, exceed 100,000 cases annually in the U.S.
- APAP is available in more than 100 over-the-counter combination formulations, including DayQuil/NyQuil Cold and Flu, Excedrin, and Robitussin Cold and Flu.
- APAP is the most commonly potential toxic pharmaceutical reported to U.S. Poison Control Centers.
- APAP toxicity is the number one cause of liver transplant in the U.S. Death rate is approximately 1 in 1000 persons. Nearly 50% of exposures occur in children ≤6 yr.
- Hepatic necrosis is most likely to occur in people who (1) are chronically malnourished, (2) have alcohol use disorder, (3) have chronic liver disease, (4) are older, and (5) use other potentially hepatotoxic medications.
- Combination opioid and acetaminophen medications are an important source of APAP toxicity, as patients are often unaware of the presence of APAP in combination forms.
- The physical examination may vary depending on the amount of time since ingestion.
- Phase I (0 to 24 h): Initial symptoms may be mild or absent and may consist of anorexia, diaphoresis, malaise, nausea, vomiting, lethargy, and a subclinical rise in transaminase levels. In a massive APAP ingestion, altered mental status and lactic acidosis can occur in phase I.
- Phase II (24 to 72 h): Right upper quadrant pain, hepatomegaly, vomiting, somnolence, tachycardia, jaundice, hypotension, and continued increase in transaminases.
- Phase III (72 to 96 h): Hepatic necrosis with abdominal pain, jaundice, hepatic encephalopathy, coagulopathy, hypoglycemia, renal failure, fatality from multiorgan failure.
- Phase IV (4 days to 2 wk): Complete resolution of symptoms and resolution of organ failure.
- Table E1 summarizes the four phases/stages of acetaminophen poisoning.
TABLE E1 Four Stages of Acetaminophen Poisoning
| Stage 1 (0-24 h) | Asymptomatic | Patients are initially asymptomatic, with normal vital signs and no physical findings. Laboratory results are normal. Nonspecific complaints of nausea, vomiting, and malaise may start to develop near the end of this stage. |
| Stage 2 (24-72 h) | Onset of hepatotoxicity | Right upper quadrant abdominal pain may develop. Levels of AST, the most sensitive indicator of hepatotoxicity, and ALT begin to rise. Later, INR values may begin to rise and renal function to deteriorate. |
| Stage 3 (72-96 h) | Maximal hepatotoxicity | The patient exhibits clinical and laboratory manifestations of hepatic necrosis: Varying degrees of hepatic encephalopathy, jaundice, renal failure, coagulation defects, and myocardial abnormalities. AST and ALT levels peak, the INR value rises, blood urea nitrogen and creatinine levels rise, and pH drops. |
| Death may occur, typically 3-5 days after overdose. Death from fulminant hepatic failure may be characterized by cerebral edema, sepsis, multisystem organ failure, hemorrhage, and acute respiratory distress syndrome. | ||
| Stage 4 (4 days-2 wk) | Recovery phase | Patients who survive stage 3 undergo complete regeneration of the liver. Laboratory abnormalities typically return to normal 5-7 days after overdose. |
ALT, Alanine transaminase; AST, aspartate transaminase; INR, international normalized ratio.
From Adams J (ed), Barton E et al (associate eds): Emergency medicine: clinical essentials, ed 2, Philadelphia, 2013, Saunders.
- The amount of APAP necessary for hepatic toxicity varies with the patient’s body size and hepatic function. APAP hepatotoxicity occurs when its typical metabolism pathway is overwhelmed, resulting in glutathione depletion and accumulation of the toxic metabolite, NAPQI. Risk factors for acetaminophen-induced hepatotoxicity are summarized in Table E2.
- When prescribing APAP, it is recommended that APAP intake should not exceed 3 to 4 g for adults and 75 mg/kg in children within a 24-h period. A potentially toxic single ingestion of APAP is 7.5 g for adults or 150 mg/kg in a child.
- There is no standard accepted definition, but a massive APAP ingestion is characterized by a single ingestion of >30 g to >50 g or an APAP concentration of greater than 250 mcg/ml to 300 mcg/ml in 4 h.
- A standardized nomogram (Fig. E1) is used to determine potential hepatic toxicity by knowing the APAP plasma level and the number of hours after ingestion. See the APAP ingestion algorithm.
Figure E1 Normal Treatment Line and High-Risk Treatment Line for the Initiation of N-Acetylcysteine (NAC) Treatment Based on Serum Acetaminophen Levels and Hours after Ingestion
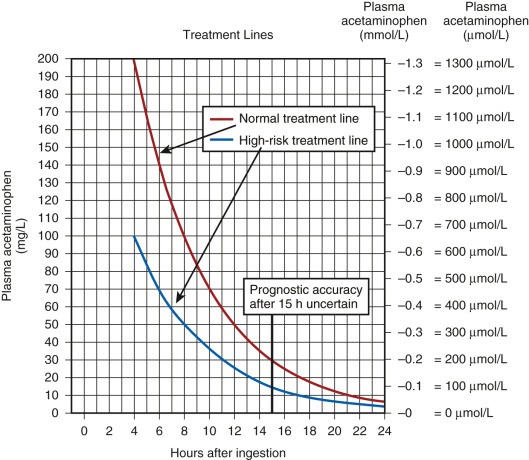
The high-risk line has been adopted as the standard and only treatment line in the United Kingdom. This is done to avoid confusion and to reduce the risk of undertreatment in patients with chronically induced hepatic enzymes, such as those receiving antiepileptic medication, chronic ethanol abusers, or smokers. If there is any doubt about the number of hours since ingestion, or if there is any suggestion of a staggered overdose or chronic overingestion, NAC should be administered.
(From Paracetamol overdose: new guidance on treatment with intravenous acetylcysteine, Drug Safety Update 6[2]:A1, 2012.)
TABLE E2 Risk Factors for Acetaminophen-Induced Hepatotoxicity
| Factor | Relevance | ||
|---|---|---|---|
| Age | Children may be more resistant than adults | ||
| Dose | Minimal hepatotoxic dose: 7.5g (100 mg/kg) in adults, 150 mg/kg in children Severe toxicity possible with dose >15 g | ||
| Blood level of acetaminophen | Influenced by dose, time after ingestion, gastric emptying Best indicator of risk of hepatotoxicity | ||
| Chronic excessive alcohol ingestion | Toxic dose threshold is lowered; worsens prognosis (also related to late presentation); nephrotoxicity common | ||
| Fasting | Toxic dose threshold is lowered-therapeutic misadventure (see text) | ||
| Concomitant medication | Toxic dose threshold is lowered-therapeutic misadventure; worsens prognosis (e.g., isoniazid, phenytoin, zidovudine) | ||
| Time of presentation | Late presentation or delayed treatment (>16 h) predicts worse outcome |
From Feldman M et al: Sleisenger and Fordtran’s gastrointestinal and liver disease, ed 11, Philadelphia, 2021, Elsevier.