Indications
- Knee joint aspiration is useful in the diagnosis of knee effusions (i.e., to diagnose crystalline disease, hemarthrosis, or rule out infection) and for the reduction of pain and pressure from effusions.
- Corticosteroid injection of the knee is indicated for the treatment of osteoarthritis, crystalline disease, or synovitis in rheumatoid arthritis.
- HA viscosupplementation injections are U.S. Food and Drug Administration (FDA)-approved for knee osteoarthritis.
- Knee injection therapy may be appropriate if knee pain continues despite evidence-based conservative therapy (activity modification, weight loss, acetaminophen, oral or topical NSAIDs, physical therapy, ice, heat).
- Strength of evidence for IA knee injection therapies is strongest for corticosteroid (Category 1A). In a recent large meta-analysis assessing pain relief at 3 mo posttreatment, IA corticosteroid had a larger effect size than IA placebo, oral placebo, oral acetaminophen, and all other oral treatments.
- Strength of evidence for HA injections is more controversial because many of the HA studies were funded by pharmaceutical companies. 2012 guidelines by ACR list HA use as no recommendation, and current American Academy of Orthopedic Surgeons recommends against HA use, due to an effect size no better than NSAIDS, with higher risk of side effects. However, anecdotally, many patients report longer relief with HA injections than corticosteroid.
- No guidelines currently recommend use of newer injectable agents including platelet-rich plasma, immunologics, or botulinum toxin because evidence is still developing.
- Concerns regarding chondrotoxic effects from lidocaine, bupivacaine, or other IA anesthetics are mainly based on cell culture, animal studies, and high-dose exposure in operating room procedures. Evidence for harm in small doses in a single IA exposure is lacking. However, if patients are concerned, the knee injections can be done without IA anesthetic agents.
- ICD10:
- M17.0 Bilateral primary osteoarthritis of knee
- M17.10 Unilateral primary osteoarthritis, unspecified knee
- M17.2 Bilateral post-traumatic osteoarthritis of knee
- M17.3 Unilateral post-traumatic osteoarthritis of knee
Anatomy
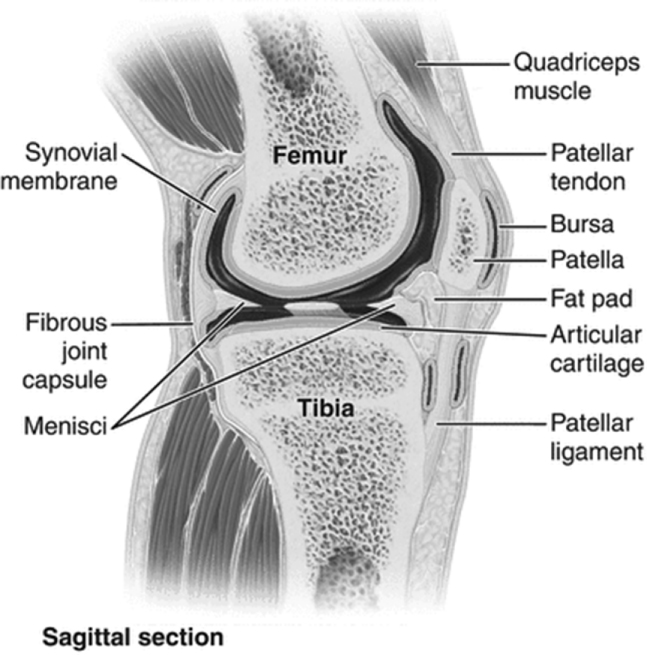
- The knee joint consists of the femoral–tibial and femoral–patellar joints, with stabilization from the anterior and posterior cruciate ligaments and the medial and lateral collateral ligaments.
Supplies
- Gloves
- Alcohol swabs
- Betadine or chlorhexidine swabs
- 18-gauge needle for drawing up medications or Medic anti-stick needle
- 3-mL syringe with 25-gauge 1-inch needle for injection
- 20- to 60-mL syringe with 18- to 22-gauge 1½-inch needle if aspirating
- 5- to 10-mL syringe with 21- to 22-gauge 1½-inch needle for injecting
- 5 to 7 mL 1% lidocaine without epinephrine and or 0.25% bupivacaine (if using)
- 1 mL 40 mg/mL (40 mg) triamcinolone suspension (Kenalog) or equivalent
- Ethyl chloride
- Gauze
- Band-Aid
Technique
- The knee joint can be aspirated or injected from multiple approaches, including the anterior medial or lateral approach with the knee flexed to 90 degrees, or a superior or midpatellar approach with the knee in extension.
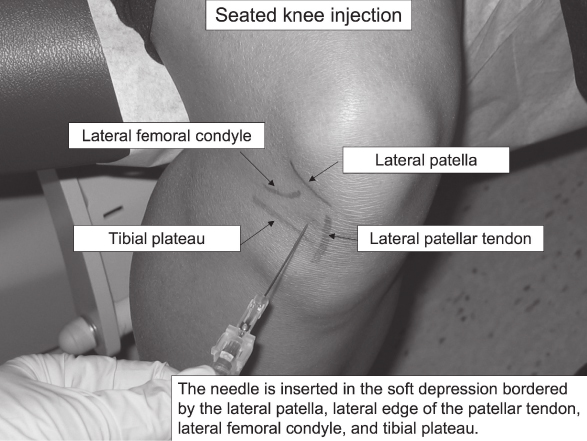
- For the anterior approach, the patient is seated with the knee flexed at 90 degrees. The needle is inserted in the “soft spot” demarcated by the patella, patellar tendon, tibial plateau, and distal femoral condyle. The needle angle is parallel to the floor and aimed slightly posterior.
- The anterior seated approach has the advantage of less bony discomfort but had a success rate of 70–75% in a trial comparing knee injection techniques. The supine lateral midpatellar approach had a higher success rate of 93% in the same study and may be a more reliable access to the knee joint in larger patients.
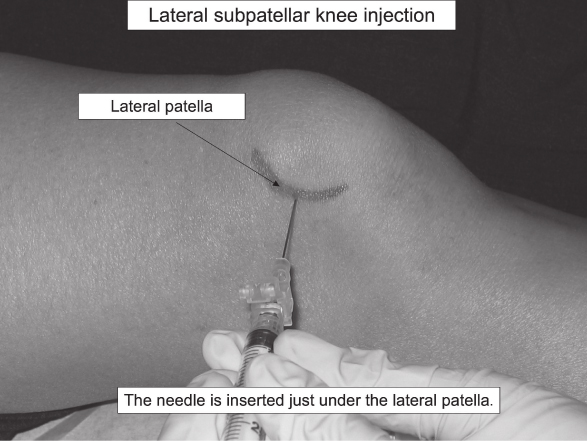
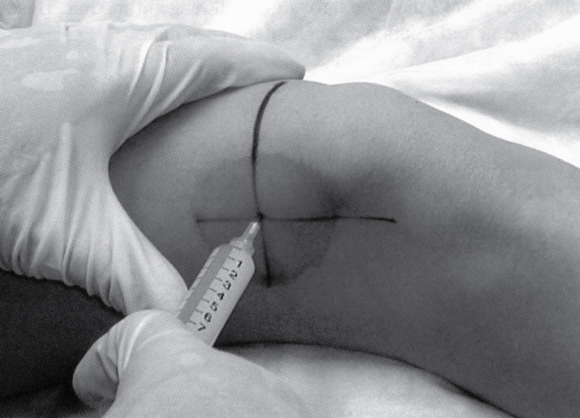
- For the supine medial or lateral midpatellar approach, position the patient’s knee either in full extension, which gives the most patellar mobility, or slightly flexed at 5 degrees with a rolled towel supporting underneath. The needle is advanced parallel to the floor directed straight toward the patellar midpole.
- For the superior approach, draw lines from the superior and lateral borders of the patella—at the intersection of these lines, insert the needle at a 45-degree angle directed toward the middle of the patella.
- After selecting an injection approach, mark the entry point with a pen or needle cap.
- Cleanse the skin with alcohol swab and then Betadine × 3 or chlorhexidine × 45 sec.
- Apply ethyl chloride for topical cooling.
- If draining an effusion, be careful not to move the needle during negative pressure aspiration.
- Keep the needle hub and syringe tip sterile during syringe transfers.
- Gentle pressure on the suprapatellar bursa may facilitate more complete drainage of the effusion.
- After aspiration, remove the aspiration syringe and connect the therapeutic syringe securely.
- Inject the therapeutic medication into the joint—there should be minimal resistance to flow.
- Remove needle and apply pressure with gauze.
- Apply Band-Aid.
Aftercare
- Lidocaine lasts 4 to 6 hr; bupivacaine can last up to 12 hr—avoid heavy exercise while the IA anesthetics are in effect because the decreased proprioceptive feedback may increase risk of falls.
- Patients may have mild discomfort at the injection site and can use ice or NSAIDs to mitigate pain until the injection takes effect.
- Inform your patient that steroids may take 2 to 3 days, and HA may take up to several weeks to take effect.
- After 3 to 5 days, the patient can resume his or her regular activity and advance as tolerated.
- Instruct patient to return to your office if they develop redness, swelling, or increased pain at the injection site.
- Recommend continued isometric quadriceps strengthening, low-impact exercise, and weight loss.
- Repeat injection can be done after 3 mo if the steroid injection afforded adequate pain relief—average duration of effect for joint steroid injections is 4 wk.
CPT Code
- 20610 Arthrocentesis, aspiration and/or injection; major joint or bursa (e.g., shoulder, hip, knee, subacromial bursa); without ultrasound guidance
- 20611 Arthrocentesis, aspiration and/or injection; major joint or bursa (e.g., shoulder, hip, knee, subacromial bursa); with ultrasound guidance, with permanent recording and reporting