Adult Dosing
Multiple Sclerosis (Relapsing)
- 300 mg IV (infused over 1 hr) q4 wks
Crohn's disease
- 300 mg IV (infused over 1 hr) q4 wks
- Do not use concomitantly with immunosuppressants or TNF-alpha inhibitors
- Discontinue therapy if no benefit occurs by 12 wks
- For patients on chronic steroid therapy, taper steroids as natalizumab benefit is seen. Complete steroid tapering by 6 months
- If steroid tapering takes more than 6 months or if patients require additional steroid use for more than 3 months in a calendar year, discontinue natalizumab
Pediatric Dosing
- Safety and effectiveness in pediatric patients have not been established
- Therapy may increase the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability [US Black Box Warning]
- At the first sign or symptom suggestive of PML, withhold therapy immediately [US Black Box Warning]
- Only prescribers registered with TOUCH program can prescribe natalizumab. Only patients enrolled for the program and meeting all the criteria can be prescribed natalizumab [US Black Box Warning]
- Increased risk of developing encephalitis and meningitis caused by herpes simplex and varicella zoster viruses have been reported with natalizumab. Closely monitor patients receiving natalizumab for signs and symptoms of meningitis and encephalitis. If herpes encephalitis or meningitis occurs natalizumab should be discontinued, and appropriate treatment for herpes encephalitis/meningitis should be administered
- Monitor patients for hypersensitivity reactions including serious systemic reactions which may occur within two hours of the start of the infusion. If a hypersensitivity reaction occurs, discontinue administration and initiate appropriate therapy
- Therapy may suppress immunity and may increase the risk for infections
- Monitor serum hepatic enzymes and total bilirubin as natalizumab may cause hepatotoxicity including markedly elevated serum hepatic enzymes and elevated total bilirubin
- Periodically monitor hematologic changes as therapy is associated with increase in circulating lymphocytes, monocytes, eosinophils, basophils, and nucleated red blood cells. It may reduce hemoglobin levels
Cautions: Use Cautiously in
- Hepatic impairment
- Immunocompromised patients
- Geriatric population (safety not established for age > 65 yrs )
Pregnancy Category:C
Breastfeeding: Safety unknown. There are no data on the use of natalizumab during breastfeeding. Discourage breastfeeding with any immunomodulatory drug or leave the decision on breastfeeding with interferon beta up to the patient. This information is based upon LactMed database (available at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT last accessed 10 January 2011)Manufacturer advises discontinuation of drug or discontinuation of breastfeeding considering the importance of the drug to the mother.
 monoclonal antibody produced in murine myeloma cells. Natalizumab contains human framework regions and the complementarity-determining regions of a murine antibody that binds to
monoclonal antibody produced in murine myeloma cells. Natalizumab contains human framework regions and the complementarity-determining regions of a murine antibody that binds to 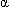 4-integrin.
4-integrin.


