Return to drug monograph
Drug Name: Pravachol 10 MG Oral Tablet
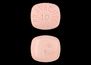
Pill Image:
Ingredient(s): Pravastatin
Imprint: P;Pravachol;10
Color(s): Pink
Shape: Rectangle
Size (mm): 6.00
Score: 1
Inactive Ingredient(s): croscarmellose sodium / lactose / magnesium oxide / magnesium stearate / microcrystalline cellulose / red ferric oxide / povidone
Drug Label Author:
Bristol-Myers Squibb
DEA Schedule:
Non-Scheduled
 [
[