Adult Dosing
Complicated intra-abdominal infections (used in combination with metronidazole)
- 2.5 g (2 g ceftazidime/0.5 g avibactam) IV infusion over 2 hours q8 hrs x 5-14 days
Complicated urinary tract Infections including pyelonephritis
- 2.5 g (2 g ceftazidime/0.5 g avibactam) IV infusion over 2 hours q8 hrs x 7-14 days
Note:
- The above dosage is recommended for patients with creatinine clearance (CrCl) >50 mL/min
- Refer package insert for preparation of the ceftazidime/avibactam solution for administration and drug compatibility
Pediatric Dosing
- Safety and effectiveness in pediatric patients have not been established
Renal Dose Adjustment (Based on CrCl)
Moderately or severely impaired renal function (CrCl  50 mL/min)
50 mL/min)
- CrCl 31-50 mL/min: 1.25 g (ceftazidime 1 g and avibactam 0.25 g) IV infusion over 2 hrs q8 hrs
- CrCl 16-30 mL/min: 0.94 g (ceftazidime 0.75 g and avibactam 0.19 g) IV infusion over 2 hrs q12 hrs
- CrCl 6-15 mL/min: 0.94 g (ceftazidime 0.75 g and avibactam 0.19 g) IV infusion over 2 hrs q24 hr
- CrCl
 5 mL/min: 0.94 g (ceftazidime 0.75 g and avibactam 0.19 g) IV infusion over 2 hrs q48 hr
5 mL/min: 0.94 g (ceftazidime 0.75 g and avibactam 0.19 g) IV infusion over 2 hrs q48 hr
Note:
- For patients with changing renal function, CrCl should be monitored at least daily and dosage of ceftazidime/avibactam adjusted accordingly
- Both ceftazidime and avibactam are hemodialyzable; hence, the medication should be administered after hemodialysis on hemodialysis days
Hepatic impairment
- Hepatic impairment: No dose adjustments
- In a cIAI clinical trial, clinical cure rates were lower in patients with baseline CrCl of 30 to
 50 mL/min compared to those with CrCl >50 mL/min. The reduction in cure rates was more in patients treated with ceftazidime/ avibactam plus metronidazole (45% cure rate) compared with meropenem-treated patients (74% cure rate). The dose of ceftazidime/avibactam was 33% lower than the dose recommended for patients with moderate renal impairment. Monitor CrCl at least daily in patients with changing renal function and adjust the dose accordingly
50 mL/min compared to those with CrCl >50 mL/min. The reduction in cure rates was more in patients treated with ceftazidime/ avibactam plus metronidazole (45% cure rate) compared with meropenem-treated patients (74% cure rate). The dose of ceftazidime/avibactam was 33% lower than the dose recommended for patients with moderate renal impairment. Monitor CrCl at least daily in patients with changing renal function and adjust the dose accordingly - Serious and occasionally fatal anaphylactic and serious skin reactions have been reported in patients receiving beta-lactam antibacterial drugs. Inquire about previous hypersensitivity reactions to other cephalosporins, penicillins, or carbapenems before initiating the therapy. Use cautiously in penicillin or other beta-lactam-allergic patients because cross sensitivity among beta-lactam antibacterial drugs has been established. Discontinue the drug if an allergic reaction occurs
- <i>Clostridium difficile</i>-associated diarrhea (CDAD) ranging in severity from mild diarrhea to fatal colitis has been reported with therapy. If CDAD is suspected or confirmed, discontinue the antibacterial drugs not directed against <i>C. difficile</i>. Manage fluid and electrolyte levels, supplement protein intake, and monitor antibacterial treatment for CDAD
- Central nervous system reactions such as seizures, nonconvulsive status epilepticus, encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia have been reported in patients treated with ceftazidime, particularly in the setting of renal impairment. Adjust dose based on CrCl
- Prescribe ceftazidime/avibactam only to those patients with proven or strongly suspected bacterial infection to avoid risk of developing drug-resistant bacteria
Cautions: Use cautiously in:
- Renal impairment
- Hypersensitivity to penicillin
- History of antibiotic associated colitis
- Seizure disorder
Pregnancy Category:B
Breastfeeding: Safety unknown. Ceftazidime: Limited information indicates that maternal doses of ceftazidime up to 2 grams produce low levels in milk that are not expected to cause adverse effects in breastfed infants. This information is based upon LactMed database (available at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT). This drug is compatible and considered safe with breastfeeding based upon data from AAP Policy Guidelines (available at http://aappolicy.aappublications.org/cgi/content/full/pediatrics;108/3/776 last accessed 06 February 2016). Manufacturer advises caution.

US Trade Name(s)
US Availability
Avycaz (ceftazidime/avibactam)
- PWDR for INJ: 2.5 g (2 g/0.5 g) vials

Canadian Trade Name(s)
Canadian Availability

UK Trade Name(s)
UK Availability

Australian Trade Name(s)
Australian Availability
 50 mL/min)
50 mL/min) 5 mL/min: 0.94 g (ceftazidime 0.75 g and avibactam 0.19 g) IV infusion over 2 hrs q48 hr
5 mL/min: 0.94 g (ceftazidime 0.75 g and avibactam 0.19 g) IV infusion over 2 hrs q48 hr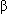 -lactamase enzymes and effectively extends the antibiotic spectrum of ceftazidime to include many gram-negative bacteria normally not susceptible to ceftazidime
-lactamase enzymes and effectively extends the antibiotic spectrum of ceftazidime to include many gram-negative bacteria normally not susceptible to ceftazidime


