- Inhibits the synthesis of
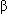 (1, 3)-D-glucan, a necessary component of the fungal cell wall.
(1, 3)-D-glucan, a necessary component of the fungal cell wall.
- Death of susceptible fungi.
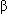 (1, 3)-D-glucan, a necessary component of the fungal cell wall.
(1, 3)-D-glucan, a necessary component of the fungal cell wall.Absorption: IV administration results in complete bioavailability.
Distribution: Widely distributed to tissues.
Protein Binding: 97%.
Metabolism/Excretion: Slowly and extensively metabolized; <1.5% excreted unchanged in urine.
Half-life: Polyphasic: 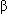 phase: 9–11 hr;
phase: 9–11 hr; 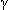 phase: 40–50 hr.
phase: 40–50 hr.
Contraindicated in:
Use Cautiously in:
Derm: flushing, pruritus, rash, STEVENS-JOHNSON SYNDROME (SJS), TOXIC EPIDERMAL NECROLYSIS.
GI: ↑liver enzymes, diarrhea, nausea, vomiting.
GU: ↑serum creatinine.
Local: venous irritation at injection site.
Neuro: headache.
Resp: bronchospasm.
Misc: chills, fever, (INCLUDING ANAPHYLAXIS AND ANGIOEDEMA)HYPERSENSITIVITY REACTIONS .
Drug-Drug:
 3 mo): 70 mg/m2 (max = 70 mg) initially followed by 50 mg/m2 once daily (max = 70 mg/day); duration determined by clinical situation and response.
3 mo): 70 mg/m2 (max = 70 mg) initially followed by 50 mg/m2 once daily (max = 70 mg/day); duration determined by clinical situation and response.Hepatic Impairment
IV Administration: