The purpose of evidence-based practice (EBP) is to use the best available evidence to make informed patient-care decisions that ultimately improve the treatment outcomes and safety of treatment for patients. How pharmacologic agents affect patients is often the subject of research; such research is required by the Food and Drug Administration (FDA) before and after drug approval. Any medication can be the subject of an evidence-based clinical review. But what does "evidence-based" mean, and how does it relate to nursing?
Evidence-based nursing practice can be viewed as a foundation of professional practice. It is an approach to making decisions, providing nursing care, and improving clinical practice based upon clinical expertise in combination with the most current and relevant research evidence. Still subject to debate are questions about the sufficiency and quality of evidence. For example, what kind of evidence is needed? How much evidence is necessary to support, modify, or change clinical practice? And were the studies reviewed of "good" quality and are their results valid?
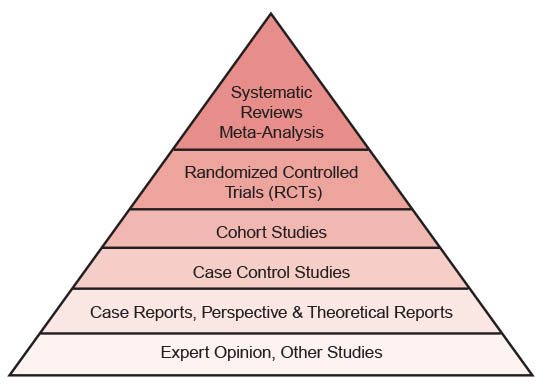
Clinicians use a hierarchy of evidence to rank types of research reports from the most valuable and scientifically rigorous to the least useful. The hierarchy makes clear that some level of evidence about the effect of a particular treatment or condition exists, even if the evidence is considered weak. Figure 1 illustrates a hierarchy of evidence pyramid with widely accepted rankings: the most scientifically rigorous at the top, the least scientifically rigorous at the bottom. Clinicians should look for the highest level of available evidence to answer their clinical questions. It is important that clinicians also apply the second fundamental principle of EBP, which is that evidence alone is not sufficient to make clinical decisions. Decision makers must always trade off the benefits and risks, as well as the costs associated with alternative treatment options, and consider the patient’s values and preferences.
Evidence-Based Practice and Its Importance in Pharmacology
Evidence-based practices in pharmacology generally are derived from well-designed randomized controlled trials (RCTs) or other experimental designs that investigate a drug’s therapeutic and nontherapeutic effects. FDA-approved pharmacologic agents have undergone rigorous testing through RCTs, but nurses have the responsibility to evaluate the findings for the best scientific evidence available and to recognize the most appropriate, safest, and efficacious drugs for their patients.
One valuable and quickly accessible resource for evaluating the current highest level of pharmacologic evidence is the Cochrane Database of Systematic Reviews. The Cochrane library and databases provide full text of high-quality, regularly updated systematic reviews, protocols, and clinical trials.
AHRQ’s Evidence-Based Practice Centers (EPCs) provide evidence reports and technology assessments that can assist nurses in their efforts to provide the highest quality and safest pharmacologic health care available. The EPCs systematically review the relevant scientific literature, conduct additional analyses (when appropriate) prior to developing their reports and assessments, and provide guideline comparisons.
Evidence-based systematic guidelines provide nurses with access to the most current knowledge, enabling them to critically appraise the scientific evidence and its appropriateness to their patient population. This is especially important given the need for nurses to keep abreast of the rapidly changing pharmacologic agents in use. New drugs are approved each month, compelling nurses to know these drugs’ intended uses, therapeutic effects, interactions, and adverse effects.
Evidence-based practice requires a shift from the traditional paradigm of clinical practice—grounded in intuition, clinical experience, and pathophysiologic rationale—to a paradigm in which nurses must combine clinical expertise, patient values and preferences, and clinical circumstances with the integration of the best scientific evidence to make conscientious, well-informed, research-based decisions that affect nursing patient care.
***
- Sally Villasenor DNP RN ACNP-BC
Nurse Practitioner and Assistant Professor Wayne State University College of Nursing Detroit Michigan
- References:
- Curtis K, Fry M, Shaban RZ, Considine J. (2016). Translating research findings to clinical nursing practice. Journal of Clinical Nursing. 2017;26:862-872. https://doi.org/10.1111/jocn.13586
- Melnyk BM, Fineout-Overholt E. (2019). Evidence-based practice in nursing & healthcare: A guide to best practice. (4th ed). Philadelphia, PA: Lippincott Williams & Wilkins. ISBN: 9781496384539
- Polit DF, Beck CT. (2021). Essentials of nursing research: Appraising evidence for nursing practice. (10th ed). Philadelphia, PA: Wolters Kluwer Health. ISBN: 9781975141851