Synonym/Acronym
pulmonary function tests (PFTs).
Rationale
To assess respiratory function to assist in evaluating obstructive versus restrictive lung disease and to monitor and assess the effectiveness of therapeutic interventions.
This Core Diagnostic Study is commonly used to identify impairment of normal lung function related to obstructions (e.g., asthma and chronic obstructive pulmonary disease [COPD] that often cause difficulty exhaling) and restrictions (e.g., pulmonary fibrosis that often causes difficulty inhaling).
Patient Preparation
There are no fluid restrictions unless by medical direction. Instruct the patient to refrain from smoking tobacco or eating a heavy meal for 4 to 6 hr prior to the study. Protocols may vary among facilities. Instruct the patient to avoid bronchodilators (oral or inhalant) for at least 4 hr before the study, as directed by the health-care provider (HCP).
Normal Findings
- Normal findings, formulas used to calculate derived measurements, and interpretation of PFTs are based on comparisons to values obtained from healthy individuals with the same physical (e.g., age, gender, height, and weight) and ethnic characteristics (where applicable) from the same general population.
- Normal respiratory volume and capacities, gas diffusion, and distribution
- No evidence of COPD or restrictive pulmonary disease
- Hypoxia occurs at oxygen saturation levels less than 90%. Significant hypoxia, levels less than 85%, require immediate evaluation and treatment.
Timely notification to the requesting HCP of any critical findings and related symptoms is a role expectation of the professional nurse. A listing of these findings varies among facilities.
(Study type: Pulmonary function tests; related body system: Respiratory system.)
Pulmonary function studies provide information about the volume, pattern, and rates of airflow involved in respiratory function. These studies may also include tests involving the diffusing capabilities of the lungs (i.e., volume of gases diffusing across a membrane). A complete pulmonary function study includes the determination of all lung volumes, spirometry, diffusing capacity, maximum voluntary ventilation, flow-volume loop, and maximum expiratory and inspiratory pressures. (See Figure 1 showing lung volumes measured during PFT.) Other studies include assessment of small airway volumes.
Figure 1: Examples of PFT Results Presented in Graphic Form
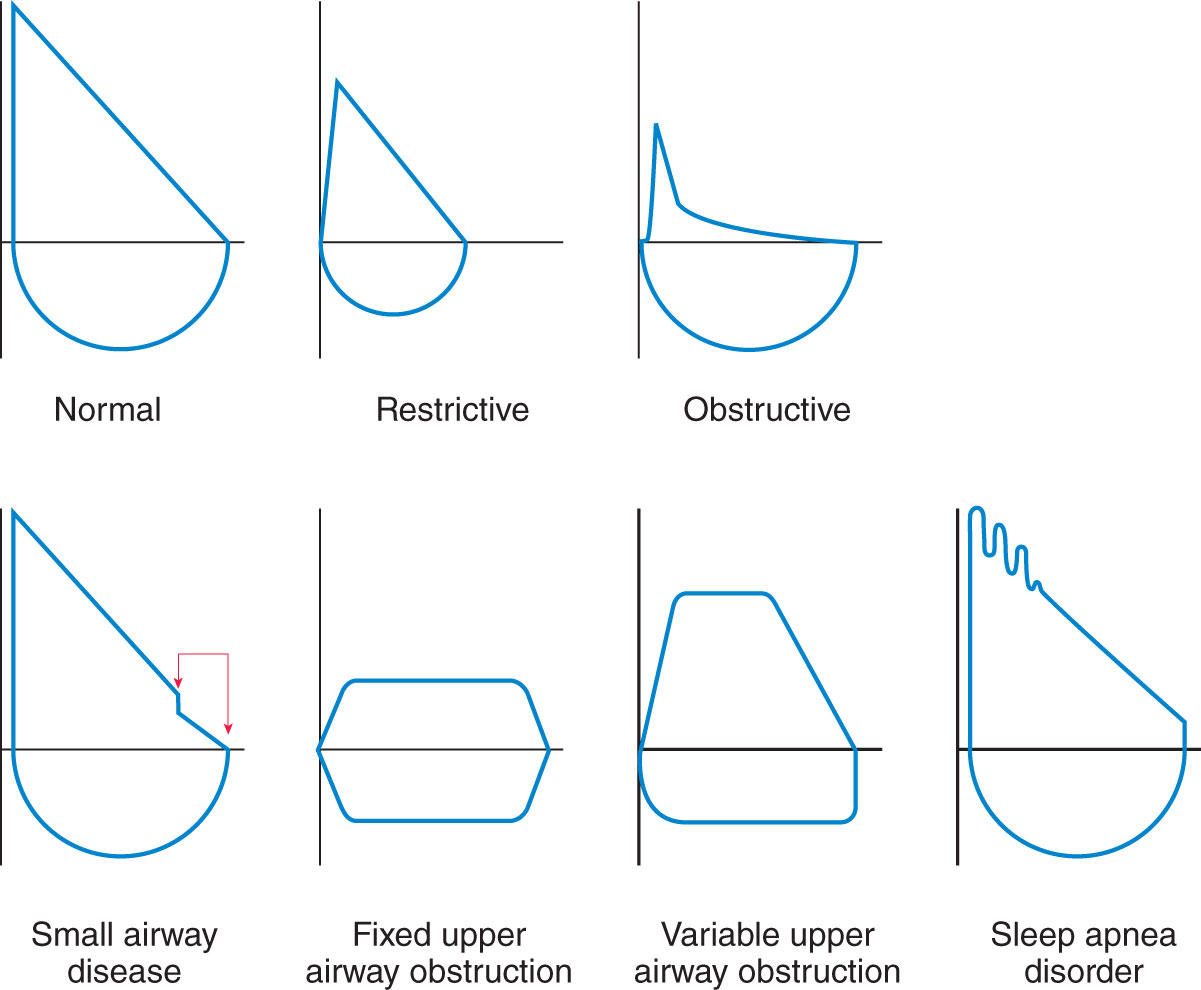
Most of the studies are conducted using a spirometer;body plethysmography is conducted in an airtight body box (plethysmograph); gas diffusion studies involve the use of equipment that measures the amount of exhaled carbon monoxide and tracer gas. The amount of air or gas breathed in and out by the patient is measured, and the results are displayed in a spirogram, plethysmogram, or by a gas diffusion measurement device.
Pulmonary function studies are classified according to lung volumes and capacities, rates of flow, and gas exchange. The exception is the diffusion test, which records the movement of a gas during inspiration and expiration. Lung volumes and capacities constitute the amount of air inhaled or exhaled from the lungs; this value is compared to normal reference values specific for the patient’s age, height, and gender. The following are volumes and capacities measured by spirometry that do not require timed testing.
| Tidal volume (TV) | Total amount of air inhaled and exhaled with one breath |
| Residual volume (RV)—best obtained directly by body plethysmography or gas dilution tests | Amount of air remaining in the lungs after a maximum expiration effort |
| Inspiratory reserve volume (IRV) | Maximum amount of air inhaled at the point of maximum expiration |
| Expiratory reserve volume (ERV) | Maximum amount of air exhaled after a resting expiration; can be calculated by the VC minus the IC |
| Vital capacity (VC) | Maximum amount of air exhaled after a maximum inspiration (can be calculated by adding the IC and the ERV) |
| Total lung capacity (TLC)—best obtained directly by body plethysmography or gas dilution tests | Total amount of air that the lungs can hold after maximum inspiration; can be calculated by adding the VC and the RV |
| Inspiratory capacity (IC) | Maximum amount of air inspired after normal expiration; can be calculated by adding the IRV and the TV |
| Functional residual capacity (FRC)—best obtained directly by body plethysmography or gas dilution tests | Volume of air that remains in the lungs after normal expiration can be calculated by adding the RV and ERV |
The volumes, capacities, and rates of flow measured by spirometry that do require timed testing include the following:
| Forced vital capacity (FVC) | Maximum amount of air that can be forcefully exhaled after a full inspiration |
| Forced expiratory volume (FEV1) | Amount of air that can be forcefully exhaled in the first second after a full inspiration (can also be determined at 2 or 3 sec) |
| Maximal midexpiratory flow (MMEF) | Also known as forced expiratory flow rate (FEF25–75), or the maximal rate of airflow during a forced expiration |
| Forced inspiratory flow rate (FIF) | Volume inspired from the RV at a point of measurement (can be expressed as a percentage to identify the corresponding volume pressure and inspired volume) |
| Peak inspiratory flow rate (PIFR) | Maximum airflow during a forced maximal inspiration |
| Peak expiratory flow rate (PEFR) | Maximum airflow expired during FVC |
| Flow-volume loops (F-V) | Flows and volumes recorded during forced expiratory volume and forced inspiratory VC procedures |
| Maximal inspiratory-expiratory pressures | Strengths of the respiratory muscles in neuromuscular disorders |
| Maximal voluntary ventilation (MVV) | Maximal volume of air inspired and expired in 1 min (may be done for shorter periods and multiplied to equal 1 min) |
Other studies for gas-exchange capacity, small airway abnormalities, and allergic responses in hyperactive airway disorders can be performed during the conventional pulmonary function study. These include the following:
| Diffusing capacity of the lungs (DL) | Rate of transfer of carbon monoxide through the alveolar and capillary membrane in 1 min. Note: Patient inhales a test mixture of air containing a small amount of carbon monoxide (CO) and a tracer gas, such as methane or helium. CO binds to Hgb with an affinity 250 times greater than oxygen |
| Closing volume (CV) | Measure of the closure of small airways in the lower alveoli by monitoring volume and percentage of alveolar nitrogen after inhalation of 100% oxygen |
| Isoflow volume (Viso) | Flow-volume loop test followed by inhalation of a mixture of helium and oxygen to determine small airway disease |
| Body/lung plethysmography | Measure of thoracic gas volume, elasticity or compliance of the lungs, and airway resistance in the respiratory tree |
| Bronchial provocation | Quantification of airway response after inhalation of methacholine |
| Arterial blood gases (ABGs) | Measure of oxygen, pH, and carbon dioxide in arterial blood |
Values are expressed in units of mL, %, L, L/sec, and L/min, depending on the test performed.
Note: See Figure 1 showing some examples of PFT results presented in graphic form; the graphs assist in interpreting the findings and establishing the diagnosis of respiratory conditions.
 Patients with cardiac insufficiency, recent myocardial infarction, and presence of chest pain that affects inspiration or expiration ability.
Patients with cardiac insufficiency, recent myocardial infarction, and presence of chest pain that affects inspiration or expiration ability.
Normal adult lung volumes, capacities, and flow rates are as follows:
| TV | 500 mL at rest |
| RV | 1,200 mL (approximate) |
| IRV | 3,000 mL (approximate) |
| ERV | 1,100 mL (approximate) |
| VC | 4,600 mL (approximate) |
| TLC | 5,800 mL (approximate) |
| IC | 3,500 mL (approximate) |
| FRC | 2,300 mL (approximate) |
| FVC | 3,000–5,000 mL (approximate) |
| FEV1/FVC | 81%–83% |
| MMEF | 25%–75% |
| FIF | 25%–75% |
| MVV | 25%–35% or 170 L/min |
| PIFR | 300 L/min |
| PEFR | 450 L/min |
| F-V loop | Normal curve |
| DCOL | 25 mL/min per mm Hg (approximate) |
| CV | 10%–20% of VC |
| Viso | Based on age formula |
| Bronchial provocation | No change, or less than 20% reduction in FEV1 |
Note: Normal values listed are estimated values for adults. Actual pediatric and adult values are based on age, height, and gender. These normal values are included on the patient’s pulmonary function laboratory report.
CV = closing volume; DCOL = diffusing capacity of the lungs; ERV = expiratory reserve volume; FEV1 = forced expiratory volume in 1 sec; FIF = forced inspiratory flow rate; FRC = functional residual capacity; FVC = forced vital capacity; F-V loop = flow-volume loop; IC = inspiratory capacity; IRV = inspiratory reserve volume; MMEF = maximal midexpiratory flow (also known as FEF25–75); MVV = maximal voluntary ventilation; PEFR = peak expiratory flow rate; PIFR = peak inspiratory flow rate; RV = residual volume; TLC = total lung capacity; TV = tidal volume; VC = vital capacity; Viso = isoflow volume. (See Figure 2.)
Figure 2: Showing Lung Volumes Measured During PFT
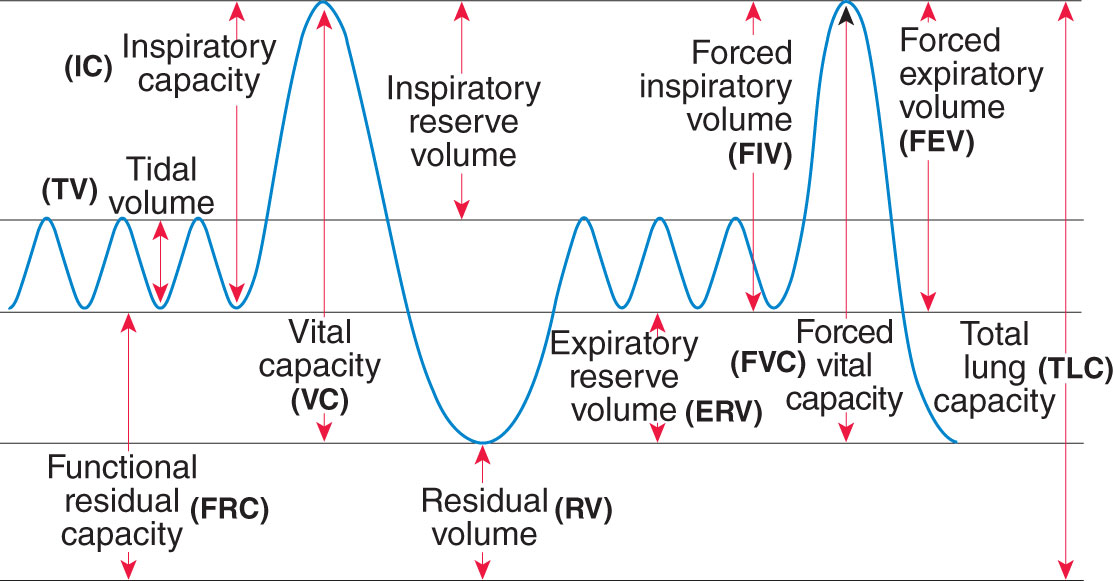
Abnormal Findings Related to
- Allergy
- Asbestosis
- Asthma
- Atelectasis
- Bronchiectasis
- Chest trauma
- COPD
- Curvature of the spine
- Myasthenia gravis
- Obesity
- Pulmonary fibrosis
- Pulmonary tumors
- Respiratory infections
- Sarcoidosis
Potential Problems: Assessment & Nursing Diagnosis/Analysis
| Problems | Signs and Symptoms |
|---|
| Activity (related to ineffective oxygenation secondary to obstruction, infection, inflammation, ineffective cardiac function) | Weakness, fatigue, chest pain with exertion, anxiety, shortness of breath, cyanosis, decreasing oxygen saturation, increased heart rate |
| Pain (related to obstruction and ischemic tissue) | Chest pain (pleuritic); increased respiratory rate; increased heart rate; fear; anxiety |
Before the Study: Planning and Implementation
Teaching the Patient What to Expect
- Review the procedure with the patient.
- Discuss how this procedure can assist in assessing lung function. Explain that the procedure takes about 45 to 60 min and is generally performed in a specially equipped room or office.
- Review the patient’s demographics (age, gender, height, and weight) for completeness; especially for lung volumes that are related to body size—standing height is an important factor.
- Explain that no discomfort will be experienced during the test. An inhalant bronchodilator will be available to treat any bronchospasms that may occur with testing.
Procedural Instructions
- Positioning for the spirometry procedure is in a sitting position on a chair near the spirometry equipment.
- Positioning for gas exchange studies is in a sitting position on a chair near the equipment that provides the labeled gas; a mask is placed over the patient’s face, and the expired gas is collected and measured.
- Positioning for the body plethysmography procedure is in a sitting position, on a chair, in an airtight body box with clear walls; it resembles a telephone booth. Notify HCP of claustrophobia.
General Instructions
- A soft clip is placed on the nose to restrict nose breathing.
- A mouthpiece is placed in the mouth, with the instruction to place lips around it to form a seal. Tubing from the mouthpiece is attached to a cylinder that is connected to a computer that measures, records, and calculates the values for the tests done.
- The patient is instructed to inhale deeply and then to quickly exhale as much air as possible into the mouthpiece. Additional breathing maneuvers are performed on inspiration and expiration (normal, forced, and breath-holding).
Additional Instructions for Body Plethysmography
- The start time of the procedure is recorded when the door to the box is closed. The patient is asked to pant rapidly and shallowly, without allowing the glottis to close.
- For compliance testing, a double-lumen nasoesophageal catheter is inserted, and the bag is inflated with air. Intraesophageal pressure is recorded during normal breathing.
After the Study: Implementation & Evaluation Potential Nursing Actions
Avoiding Complications
- Monitor the patient for complications related to the procedure.
Treatment Considerations
- Follow postprocedure vital sign and assessment protocol.
- Allow the patient to rest as long as needed to recover. Resume the usual diet and medications, as directed by the HCP.
Activity
- Interventions/actions related to activity include the following: Identify normal activity patterns. Enforce activity restrictions as necessary to rest the heart and conserve oxygen. Administer ordered oxygen and explain the importance of wearing oxygen with activity. Pace activity to improve tolerance. Monitor and trend vital signs. Consider discussing the effects of altered cardiopulmonary status on sexual activity.
Pain
- Interventions/actions related to pain management include the following: Assess pain character, location, duration, and intensity using an easily understood pain rating scale. Place in a position of comfort. Administer ordered oxygen, analgesics, anticoagulants, or thrombolytics. Consider alternative measures for pain management (imagery, relaxation, music, etc.).
Safety Considerations
- Assess the patient for dizziness or weakness after the testing.
Clinical Judgement
- Consider how to overcome cultural barriers toward therapeutic recommendations to improve respiratory health.
Follow-Up Evaluation and Desired Outcomes
- Understands that depending on the results of this procedure, additional testing may be performed to evaluate or monitor disease progression and determine the need for a change in therapy.
- Acknowledges information provided for smoking cessation, as appropriate.
- Understands pathophysiology and therapeutic options related to diagnosis.

 Patients with cardiac insufficiency, recent myocardial infarction, and presence of chest pain that affects inspiration or expiration ability.
Patients with cardiac insufficiency, recent myocardial infarction, and presence of chest pain that affects inspiration or expiration ability. 