Intensive care units (ICUs) provide high-resolution and high-frequency surveillance for patients who are critically ill to capture disease trajectory in real time. This intensive monitoring enables clinicians to alter, add, or remove interventions to match the progression of disease, thereby reducing mortality, morbidity, and length of stay.
Intensive monitoring in the ICU promotes a culture of safety and provides the data needed to develop thresholds for initiating, escalating, deescalating, and ceasing treatment. Whether by invasive or noninvasive means, this monitoring aims to accurately capture disease progression.
Respiratory monitoring, invasive and noninvasive, and subsequent treatment are the mainstays of critical care; it is what separates the subspecialty from all others.
This chapter discusses the core aspects of respiratory monitoring in the ICU setting and how to troubleshoot a shift in a patient’s respiratory trajectory.
The chief focus of respiratory monitoring is on the delivery and removal (exchange) of gases, oxygen (O2), and carbon dioxide (CO2), respectively.
- Hypoxia is the condition of inadequate oxygen. Hypoxia can occur in the whole body (generalized hypoxia) or regionally (tissue hypoxia).
- Reasons for hypoxia include the following:
- Hypoxemia
- Ischemia: a deficiency in oxygen supply due to insufficient blood flow
- Histotoxic: inability of cells to use oxygen despite appropriate delivery (eg, cyanide toxicity)
- Anemia
- Inhibition of the function of hemoglobin such as in carbon monoxide poisoning or elevated methemoglobinemia
- Hypoxemia is low partial pressure of O2 in the arterial blood (Pao2) resulting in a reduction of oxygen in the arterial blood. The Pao2 should always be interpreted in relation to the level of supplemental oxygen or Fio2 (fraction of inspired oxygen).
- For example, a Pao2 of 95 mm Hg breathing 100% oxygen is quite different from a Pao2 of 95 mm Hg breathing air (21% oxygen).
- The relationship between Pao2 and Fio2 is often captured in the Pao2/Fio2 (P/F) ratio and is used to determine severity of lung injury, specifically acute respiratory distress syndrome (ARDS). A low P/F ratio reflects poor oxygen exchange, for example.
- Causes of hypoxemia include the following:
- Pulmonary diseases or conditions resulting in increased passage of deoxygenated blood from the right side of the heart to the left without participating in gas exchange
- Increased shunt (Q'S/Q'T)
- Ventilation-perfusion (V'/Q') mismatch
- A low mixed venous Po2 (eg, decreased cardiac output) will magnify the effect of shunt on Pao2.
- Hypoventilation
- Diffusion defect
- Pao2 is also decreased with decreased inspired oxygen (eg, at high altitude).
- Anoxia: complete deprivation of oxygen
- Hyperoxemia (increased Pao2) may occur when breathing supplemental oxygen. The Pao2 also increases with hyperventilation.
- Ventilation/CO2removal:Arterial partial pressure of CO2 (Paco2) is the balance between carbon dioxide production (V'co2) and alveolar ventilation (V'a).
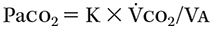
- Dead space: Portions of the airways that do not participate in gas exchange. Physiologic dead space (VDphys) is considered the TOTAL dead space and is the sum of the anatomic dead space (VDana) and the alveolar dead space (VDalv).
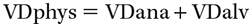
- Anatomic dead space comprises airways known to be devoid of gas exchange mechanisms—areas without alveoli (ie, trachea, bronchi).
- Anatomic dead space in an adult is usually considered 150 mL (and in children or small adults, ~2 mL/kg).
- Alveolar dead space is ventilated alveoli that lack concomitant perfusion, essentially leading to ventilation (gas flow) with reduced CO2 removal.
- This occurs whenever the pulmonary vasculature blood flow is lower than the level of gas exchange in the corresponding alveolar space. The extreme of dead space ventilation is cardiac arrest (all V' and no Q'); but a pulmonary embolism (PE) is the pathophysiology more commonly encountered.
- Note: Be mindful of added dead space from inappropriate additions to the airway circuit, which manifests greater relative impact in patients with smaller lung volumes (eg, pediatric, patients with severe kyphoscoliosis and achondroplasia).
- Dead space, total (V'DS/V'T) can also be calculated from the Bohr equation by calculating the discrepancy between arterial blood gas (ABG)-based Paco2 and end-tidal CO2 (ETCO2), which measures the ratio of dead space to total ventilation:
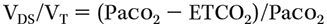
- The normal VDS/VT is 0.3 to 0.4, so 30% to 40% of the total tidal volume.
- Minute ventilation:
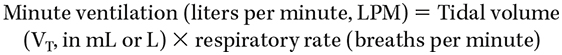
- Minute ventilation (LPM) can also be framed as the sum of physiologic dead-space minute ventilation + alveolar minute ventilation.
- Multiplying the respiratory rate (RR) by the dead-space volume determined by the Bohr equation results in the minute ventilation of the respective volume.
- For example, a physiologic dead space (or VDS) of 200 mL (0.33 of a total VT of 600 mL) at an RR of 14 = 2.8 LPM of dead-space minute ventilation; and conversely, the alveolar minute ventilation of the same patient receiving 600 mL VT results from subtracting the dead-space volume from the total (600 mL - 200 mL = 400 mL) × RR of 14 = alveolar minute ventilation of 5.6 LPM. The total minute ventilation, as displayed on the ventilator, would be 8.4 LPM.
where K is a constant.
where VDS is the volume of dead space and VT is the total tidal volume.