Nonprescription or OTC drugs are those that are safe and effective for self-medication by consumers. Pursuant to regulations finalized in 1999 with the intent to make OTC drug labeling more "user friendly" the label of a nonprescription drug must contain in part the following information (see 64 Fed. Reg. 13254; 21 C.F.R. part 201 (A, C); https://www.fda.gov/regulatory-information/search-fda-guidance-documents/labeling-otc-human-drug-products-questions-and-answers; 21 C.F.R. § 201.66):
The name and address of the manufacturer, packer, or distributor
Location of expiration date
Control numbers such as lot or batch code
Principal display panel
A statement of the identity of the product, including the established name of the drug if any, followed by an accurate statement of the general pharmacological category of the drug or principal intended action(s) (e.g., Suphedrin, pseudoephedrine hydrochloride, nasal decongestant)
The net quantity of the contents of the package
Declaration of the presence of FDandC Yellow No.5 and/or FDandC Yellow No. 6
Tamper-evident labeling
Cautions and warnings needed to protect the consumer such as what to do if an overdose occurs
Adequate directions for use (as discussed previously)
A "Drug Facts" panel (Figure 2-1) containing the following information in the following order (21 C.F.R. § 201.66):
Active ingredient(s) (including dosage unit and quantity per dosage unit)
Purpose (general pharmacological category or principal intended action)
Uses (indications)
Warnings (including the following subheadings in bold type):
"For external use only" (for topical products) or "For rectal (or vaginal) use only" for products intended for these uses
Do not use (listing of all contraindications)
Ask a doctor before use if you have (listing of all conditions and situations when the product should not be used)
Ask a doctor or pharmacist before use if you are (listing of all drug-drug and drug-food interactions)
When using this product (listing of possible side effects and substances or activities to avoid)
Stop use and ask a doctor if (listing of signs of toxicity and other reactions requiring immediate discontinuation)
"If pregnant or breastfeeding" warning
"Keep out of reach of children" and accidental overdose/ingestion warning
Directions
Other information (as required by the monograph, by regulation, or in the approved labeling)
Inactive ingredients (listed in alphabetical order)
Questions? or Questions and Comments (followed by a telephone number)
Figure 2-1: Drug facts label.
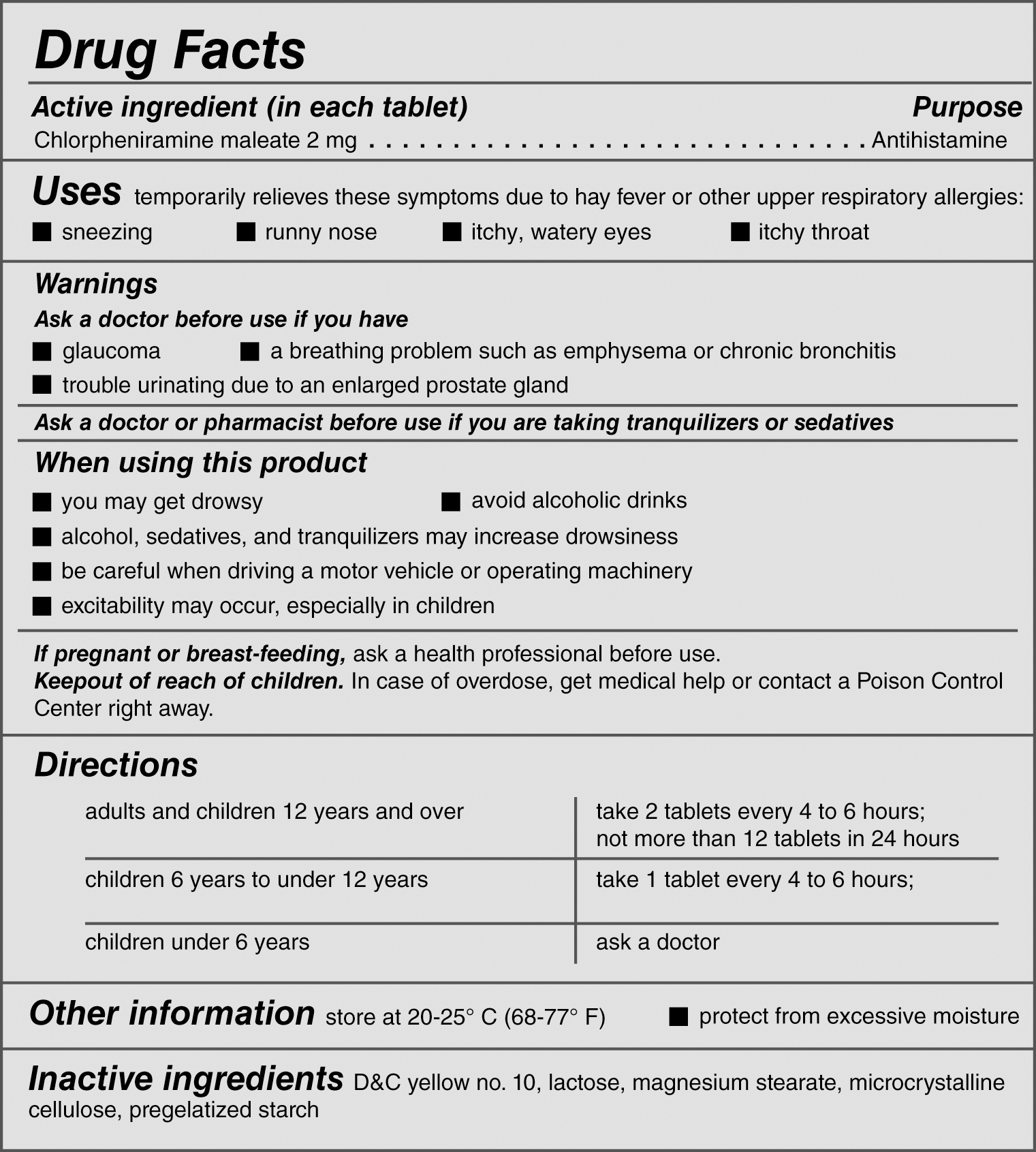
Reproduced from the U.S. Food and Drug Administration, OTC Drug Facts Label, http://www.fda.gov/drugs/resourcesforyou/consumers/ucm143551.htm
Regulations (21 C.F.R. § 201.5) further require adequate directions for use to contain:
The normal dose for each intended use and the doses for individuals of different ages and different physical conditions
The frequency and duration of administration or application
The administration or application in relation to meals, onset of symptoms, or other time factors
The route or method of administration or application
Any required preparation for use
The regulations provide that OTC drug labels must be easy to read and easy to understand as well as be of a minimum size type. These format requirements are designed to make it easier for consumers to select the appropriate product and help them use the product more effectively.
Pharmacists who repackage or relabel OTC drugs for resale must comply with the same labeling requirements as manufacturers.
Drugs That Are Both OTC and Prescription
The issue of adequate directions for use labeling also explains why some drugs are both OTC and prescription. With these drugs, the FDA has made the determination that the drug can be labeled with adequate directions for use for some indications but not others. For example, meclizine is sold OTC for the indications of nausea, vomiting, and dizziness associated with motion sickness. The drug is sold by prescription with the added indication of being possibly effective for vertigo associated with diseases affecting the vestibular system. It also explains why some drugs such as ibuprofen are OTC at one strength and prescription at other strengths. The 200 mg OTC ibuprofen carries the indication for mild to moderate pain, whereas the higher strengths prescription ibuprofen add indications of rheumatoid arthritis and osteoarthritis. (A drug can also be both OTC and prescription, depending on how it is switched from prescription to OTC status.)