- Red top or SST gel barrier tube
- 5-10 mL of venous blood
- ELISA is the most commonly used and widely available screening test for HIV infection as it is relatively simple, highly sensitive, and suitable for testing large numbers of samples
- Principle and methodology:
- ELISA may be run in a qualitative or quantitative format
- Qualitative results provide a simple positive or negative result for a sample
- In quantitative ELISA, the optical density or fluorescent units of the sample is interpolated onto a standard curve, which is typically a serial dilution of the target
- A person's serum is diluted and applied to a plate coated with HIV antigens. Antibodies to HIV if present, bind to the HIV antigens.
- A secondary antibody, which is chemically linked to an enzyme,is added that binds to the human antibodies. A substrate is applied, which reacts with the enzyme leading to a change in color or fluorescence
- The conditions where ELISA is used as a screening test for HIV include:
- In persons suspected to be infected with HIV
- In sexual partners of HIV infected persons
- In infants born to HIV infected mothers
- In IV drug users and recreational drug users
- As a part of pregnancy workup, regardless of the risk
- Should be tested as early as possible during each pregnancy
- Second HIV test during the third trimester, preferably <36 weeks, may be considered for all pregnant women
- Second HIV test in the third trimester is recommended for women with at least one of the following criteria:
- Those who receive health care in places with high incidence of HIV infection or AIDS among women aged 15 to 45 yr.
- Those who receive health care in settings where prenatal screening identifies at least one HIV-infected pregnant woman for every 1000 women screened
- Women known to be at high risk of acquiring HIV
- Women who show signs and symptoms of acute HIV infection
- Before any surgery
- For blood and blood products that will be used for transfusion
- For military personnel on active duty and all members of the reserve and National Guard (twice a year)
- For tissue and organs used for transplantation
- In all aspiring immigrants (all applicants for U.S. residence)
- In healthcare workers with direct exposure to blood during work
- In people attending STD clinics
- In people with signs and symptoms of unusual pneumonia, skin lesions, and mononucleosis-like syndrome
- Diagnostic testing to establish HIV infection may be indicated when there is a strong clinical suspicion, supported by one or more of the following clinical findings [Centers for Medicare & Medicaid Services]:
- A documented, otherwise unexplained, AIDS-defining or AIDS-associated opportunistic infection
- Another documented sexually transmitted disease, which identifies significant risk of exposure to HIV and the potential for an early or subclinical infection
- Documented acute or chronic hepatitis B or C infection that identifies a significant risk of exposure to HIV and the potential for an early or subclinical infection
- Documented AIDS-defining or AIDS-associated neoplasm
- A documented AIDS-associated neurologic disorder or otherwise unexplained dementia
- Another documented AIDS-defining clinical condition, or a history of other severe, recurrent, or persistent conditions which suggest an underlying immune deficiency (for example, cutaneous or mucosal disorders)
- Otherwise unexplained generalized signs and symptoms suggestive of a chronic process with an underlying immune deficiency (for example, fever, weight loss, malaise, fatigue, chronic diarrhea, failure to thrive, chronic cough, hemoptysis, shortness of breath, or lymphadenopathy).
- An otherwise unexplained laboratory evidence of a chronic disease process with an underlying immune deficiency (for example, anemia, leukopenia, pancytopenia, lymphopenia, or low CD4+ lymphocyte count).
- Signs and symptoms of acute retroviral syndrome with fever, malaise, lymphadenopathy, and skin rash.
- Documented exposure to blood or body fluids known to be capable of transmitting HIV (for example, needlesticks and other significant blood exposures) and antiviral therapy is initiated or anticipated to be initiated.
- Is undergoing treatment for rape (HIV testing is a part of the rape treatment protocol).
- Positive test for HIV antibody cannot determine whether patient harbors actively replicating virus or when the patient will manifest signs and symptoms of AIDS
- Treatment is more effective and less toxic when begun early in the course of HIV infection
- Infection with HIV-2 is more prevalent in West Africa and HIV-1 in the U.S, Western Europe and rest of the world
- HIV infection is described as a continuum of stages that range from the acute, transient, mononucleosis-like syndrome associated with seroconversion to asymptomatic HIV infection to symptomatic HIV infection and, finally, to AIDS, which is the end-stage of HIV infection
- Sensitivity and specificity of ELISA: > 99%
- Frequency of false-positive tests: 0.0007%
- Frequency of false-negative tests: 0.0003%
- A positive test is associated with viral replication and appearance of HIV antibodies such as IgM, IgG
- ELISA, if tested positive, should be repeated in duplicate using the same blood sample on different ELISA test. If repeatedly reactive, follow-up tests using Western blot should be done for confirmation
- Recent CDC recommendations are for HIV screening using the ELISA test for all patients aged 13-64 years, in all healthcare settings after consenting the patient for the test [Unless the patient declines (opt-out screening)]
- No specific signed consent is required for HIV testing (Many institutions still require this; check your institutional policy); General informed consent for medical care is sufficient.
- When a patient declines the HIV test, it should be documented in the patient's medical record
- Patients at high risk for HIV infection should be screened at least once every year
- All patients starting TB therapy should be routinely screened for HIV infection
Related laboratory tests:
- CD4/CD8 lymphocyte ratio
- HIV viral load
- HIV Western Blot testing
- Plasma RNA test for HIV
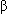 2-Microglobulin
2-Microglobulin- Complete blood count
- Cytomegalovirus antibodies
- Epstein Barr Virus antibodies
- Hepatitis B antibody and antigen
- Hepatitis C antibody
- Human T-cell lymphotropic virus types I and II
- Monospot
- Skin culture
- Syphilis serology
Additional information:
Detuned ELISA is a relatively new test used only after HIV antibodies are confirmed by Western Blot test. This test determines if the HIV infection is recent i.e., within the last six months, which is useful for deciding upon possible early treatment options
Consult your laboratory for their normal ranges as these may vary somewhat from the ones listed below.
Normal result is:
- Negative for antibodies against HIV 1 and 2 by ELISA
Factors which may result in false negative test results include:
- Agammaglobulinemia
- B-Lymphocyte dysfunction with severe HIV infection
- Bone marrow transplantation
- Common variable immunodeficiency
- End-stage AIDS
- Exchange transfusion
- Heat inactivation
- HIV group O virus
- Hypogammaglobulinemia
- Immunosuppressive therapy
- Malignancy
- Testing during the "window period" before seroconversion (Primary HIV)
A positive ELISA test indicates the possibility of an HIV infection
Factors which can rarely lead to a false positive test result include:
- Administration of human immunoglobulin preparations pooled before 1985
- Alpha interferon therapy in hemodialysis patients
- Antibody against human tissues such as
- Anti-carbohydrate antibodies
- Anti-collagen antibodies
- Anti-lymphocyte antibodies found in
- Africans
- Gay men
- Hemophiliacs
- People with leprosy
- Anti-microsomal antibodies
- Anti-mitochondrial antibodies
- Anti-nuclear antibodies
- Anti-parietal cell antibody
- Anti-smooth muscle antibody
- Anti-Hepatitis A Virus IgM
- Anti-Hepatitis B Core IgM
- Anti-HLA Class II antigens such as HLA-DR4, HLA-DQw3
- Anti-HLA I antibody
- Autoimmune diseases
- Dermatomyositis
- Systemic lupus erythematosus
- Scleroderma
- Cross-reacting viral infections such as HTLV I/II
- Epstein-Barr virus
- Heat treated specimen
- Hemolyzed serum
- Hemophilia
- Herpes simplex I
- Herpes simplex II
- Hyperbilirubinemia
- Hypergammaglobulinemia
- Infection with Mycobacterium avium
- Influenza
- Leprosy
- Lipemic serum
- Lyme disease
- Malaria
- Malignancies
- Multiple blood transfusions
- Multiple myeloma
- Organ transplantation
- Passively acquired antibody such as
- Hepatitis B immune globulin
- Transplacental HIV-1 antibody
- Pregnancy
- Primary biliary cirrhosis
- Primary sclerosing cholangitis
- Proteins on the filter paper
- Q-fever with associated hepatitis
- Recent viral infection or exposure to viral vaccines
- Renal failure/ Hemodialysis
- Rheumatoid arthritis
- Severe liver disease
- Alcoholic liver disease
- Hepatitis
- Stevens-Johnson syndrome
- Syphilis (serology positive)
- Tuberculosis
- Visceral leishmaniasis
- Centers for Disease Control: Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings. MMWR September 22, 2006 / 55(RR14);1-17. [Homepage on the Internet. Last reviewed on December 12, 2006. Last accessed on November 27, 2006. Available at URL: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm
- Centers for Medicare & Medicaid Services. NCD for Human Immunodeficiency Virus (HIV) Testing (Diagnosis) (190.14). [Homepage on the Internet]©2005. Last updated on June 19, 2006. Last accessed on December 1, 2006. Available at URL: http://www.cms.hhs.gov/mcd/viewncd.asp?ncd_id=190.14&ncd_version=2&basket=ncd%3A190%2E14%3A2%3AHuman+Immunodeficiency+Virus+%28HIV%29+Testing+%28Diagnosis%29
- Constantine NT et al. HIV testing technologies after two decades of evolution. Indian J Med Res. 2005 Apr;121(4):519-38.
- HIV InSite®. HIV Antibody Assays. [Homepage on the Internet]©2006. Last updated in May, 2006. Last accessed on November 25, 2006. Available at URL: http://hivinsite.ucsf.edu/InSite?page=kb-02-02-01
- Loussert-Ajaka I et al. HIV-1/HIV-2 seronegativity in HIV-1 subtype O infected patients. Lancet. 1994 Jun;343(8910):1393-4
- MedlinePLus Medical Encyclopedia®. HIV ELISA/Western blot. [Homepage on the Internet]©2005. Last updated on March 6, 2006. Last accessed on November 14, 2006. Available at URL: http://www.nlm.nih.gov/medlineplus/ency/article/003538.htm
- Novack L et al. Use of seroconversion panels to estimate delay in detection of anti-human immunodeficiency virus antibodies by enzyme-linked immunosorbent assay of pooled compared to singleton serum samples. J Clin Microbiol. 2006 Aug;44(8):2909-13.
- Padeh YC et al. Common variable immunodeficiency and testing for HIV-1. NEJM. 2005 Sep 8;353(10):1074-5.
- Sheikh AA et al. High frequency of false positive results in HIV screening in blood banks. J Pak Med Assoc. 2006 Jan;56(1 Suppl 1):S72-5.
- Simonney M et al. B-cell immune responses in HIV positive and HIV negative patients with tuberculosis evaluated with an ELISA using a glycolipid antigen. Tuberculosis (Edinb). 2006 Oct 5; [Epub ahead of print].
- WeberBet al.Multicenter evaluation of a new 4th generation HIV screening assay Elecsys HIV combi. Clin Lab. 2006;52(9-10):463-73.