Flowchart for the Therapy of Newly Diagnosed Acute Myeloid Leukemia (AML) 
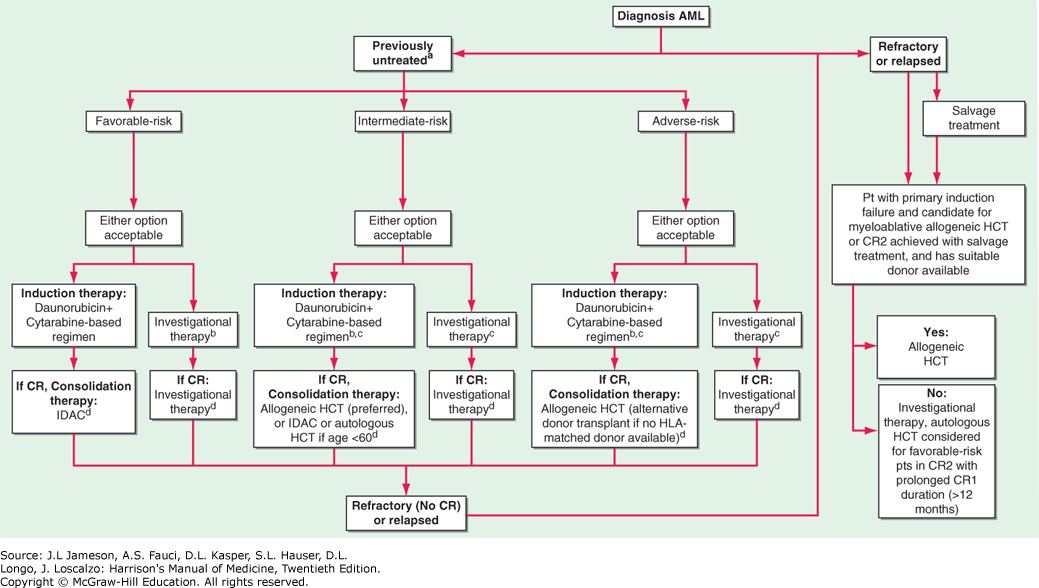
Flowchart for the therapy of newly diagnosed acute myeloid leukemia (AML). aRisk stratification according to the European LeukemiaNet. bYounger pts (<60-65 years) should routinely be offered investigational therapy on a backbone of standard chemotherapy for induction and consolidation. cOlder pts, especially those >65 years or with adverse-risk disease, or those who are unfit for intensive daunorubicin + cytarabine regimens, may be considered for investigational therapy alone or in combination with lower intensity chemotherapy regimens (azacitidine, decitabine). dInvestigational therapy as maintenance should be considered if available (after consolidation for younger pts and older pts with favorable-risk disease, and for all other older pts after induction).
For all forms of AML except acute promyelocytic leukemia (APL), standard induction therapy includes a regimen based on a 7-day continuous infusion of cytarabine (100-200 mg/m2/d) and a 3-day course of daunorubicin (60-90 mg/m2/d) with or without additional drugs. Idarubicin (12 mg/m2/d) could be used in place of daunorubicin (not shown). The value of postremission/consolidation therapy for older pts (>60 years) who do not have favorable-risk disease is uncertain. Pts who achieve complete remission (CR) undergo postremission consolidation therapy, including sequential courses of intermediate-risk cytarabine, allogeneic HCT, autologous HCT, or novel therapies, based on their predicted risk of relapse (i.e., risk-stratified therapy). Pts with APL (see text for treatment Myeloid Leukemias, Myelodysplasia, and Myeloproliferative Syndromes) usually receive tretinoin and arsenic trioxide-based regimens with or without anthracycline-based chemotherapy and possibly maintenance with tretinoin. HCT, hematopoietic cell transplantation; HLA, human leukocyte antigen; IDAC, intermediate dose cytarabine.