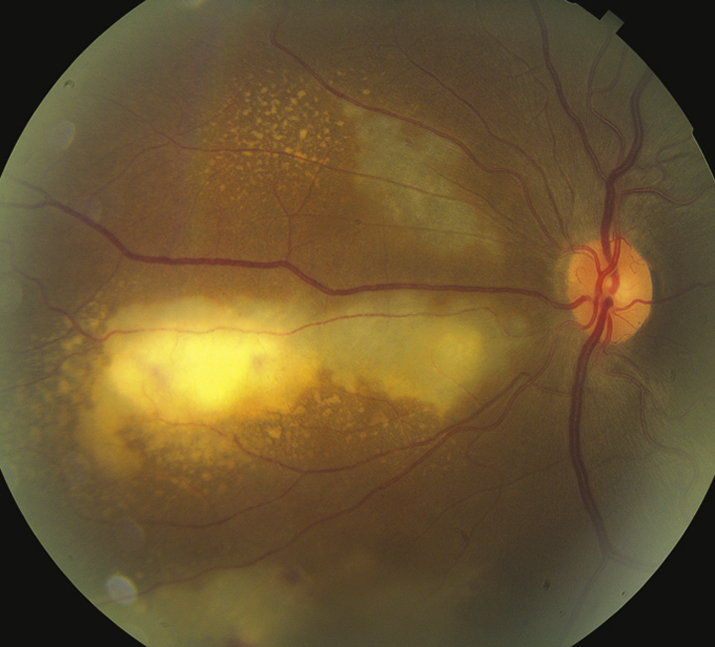
Scotoma or decreased vision in one or both eyes, floaters, or photopsias. Pain and photophobia are uncommon. Often asymptomatic.
See Table 12.9.1 for treatment details.
- Oral therapy with valganciclovir 900 mg p.o. b.i.d. for induction (21 days), followed by 900 mg p.o. daily for maintenance. Alternatively, intravenous ganciclovir 5 mg/kg b.i.d. or foscarnet 90 mg/kg b.i.d. (adjusting for renal function) may be used, followed by oral therapy valganciclovir (900 mg p.o. b.i.d. to complete 3-week induction, then 900 mg p.o. daily). Patients with progression of retinitis despite induction or who have disease that threatens the macula may benefit from intravitreal antiviral injections, but systemic therapy is still necessary to prevent the involvement of the fellow eye. The goal of treatment is quiescent retinitis (nonprogressive areas of RPE atrophy with a stable opacified border).
- Under the direction of an internist or infectious disease specialist, HAART should be initiated or optimized; immune recovery with sustained CD4+ counts >200 cells/mm3 results in decreased risk of retinal detachment, second-eye involvement, antiviral resistance, and mortality. Also, monitor for toxicity of anti-CMV therapy (valganciclovir and ganciclovir cause bone marrow toxicity; foscarnet is nephrotoxic and can cause electrolyte abnormalities and seizures; intravenous ganciclovir and foscarnet require placement of an indwelling catheter, which may cause line infection and sepsis).
- Small, macula-sparing RRDs may be treated with laser demarcation, but multiple retinal breaks are typical and may be missed; pars plana vitrectomy with silicone oil is indicated for most detachments, especially those involving the macula.
- Primary prophylaxis (prevention of CMV retinitis) with oral valganciclovir in high-risk patients is usually not recommended because of potential toxicity except in transplant patients.
12-9.1 Therapy for CMV Retinitis
| Drug | Dosing | Toxicities | Contraindications |
|---|
| Ganciclovira | Induction: 5 mg/kg i.v. b.i.d. for 14 days
Maintenance: 5 mg/kg i.v. daily | Neutropenia,b thrombocytopenia, anemia;
Discontinue nursing | Absolute neutrophil count <500/mm3, platelets <25,000/mm3;
Potentially embryotoxic | | Valganciclovir | Induction: 900 mg p.o. b.i.d. for 21 days
Maintenance: 900 mg p.o. daily | Neutropenia,b thrombocytopenia, and anemia;
Discontinue nursing | Absolute neutrophil count <500/mm3, platelets <25,000/mm3;
Potentially embryotoxic | | Foscarnet | Induction: 90 mg/kg i.v. b.i.d. twice/wk
Maintenance: 90 to 120 mg/kg i.v. dailyc (monitor creatinine and electrolytes; adjust dosing as needed) | Renal impairment neutropenia, anemia, and electrolyte imbalances | Use caution with renal impairment or electrolyte imbalances | | Cidofovir | Induction: 5 mg/kg i.v. weekly for 3 wks maintenance: 3 to 5 mg/kg i.v. every 2 wks | Dose- and schedule-dependent nephrotoxicity, hypotony (necessitates discontinuation), iritis (steroid responsive);
Must be given with probenecid | Recurrent uveitis, moderate to severe kidney disease, intolerance to probenecid |
|
a Compared with intravitreal therapy alone, there is a decreased risk of mortality (50%), systemic disease (90%), and fellow eye involvement (80%) in patients treated with systemic anti-CMV therapy. Moreover, the risk of retinitis progression is significantly greater in eyes treated with intravitreal therapy alone.
b Compared with i.v. ganciclovir, there is an increased risk of systemic disease (30%) and fellow eye involvement (50%) after 6 months. However, the relapse-free interval is greatly increased.
c During the induction phase, 500 mL of normal saline is used for each dose. During maintenance, 1000 mL of saline should be used.
- Ganciclovir resistance (reflected by positive blood or urine CMV cultures) may occur with prolonged treatment.
- All currently available anti-CMV therapy is virostatic, not virocidal, and almost all patients eventually relapse if not treated with HAART. Serial fundus photographs helpful for comparison.
- Relapse is defined as recurrent or new retinitis, movement of opacified border, or expansion of the atrophic zone.
- Relapse can occur from resistance or subtherapeutic intraocular drug levels. Reinduction with the same medication is the first line of treatment.
- Clinical resistance is defined as persistent or progressive retinitis despite induction level medication for 6 weeks. Laboratory confirmation is possible for low-grade UL97 (viral phosphotransferase) or high-grade UL54 (viral DNA polymerase) mutations.
- If resistance is recognized, a change in therapy from one antiviral to the other is indicated. Consider intravenous cidofovir 5 mg/kg once weekly for 2 weeks and then 3 to 5 mg/kg every 2 weeks. Cidofovir itself may cause uveitis and renal impairment and must be given with probenecid to reduce the nephrotoxicity. Intravitreal cidofovir is contraindicated because of the high risk of uveitis and hypotony. Cross-resistance can occur since all three anti-CMV drugs are CMV DNA polymerase inhibitors.
- Discontinuation of anti-CMV maintenance therapy may be considered in select patients receiving HAART who have CD4+ counts >100 cells/mm3 for greater than 6 months and completely quiescent CMV retinitis. In these patients, whose immune system can control CMV, stopping maintenance therapy may prevent drug toxicity and drug-resistant organisms. In iatrogenically immunosuppressed patients, cessation or reduction in dosage of immunosuppressive drugs may be required for long-term control of CMV retinitis.
- Immune recovery uveitis (IRU): Occurs in previously immunocompromised patients (HIV/iatrogenic) with CMV after the CD4+ count or immune system reconstitutes. In the presence of a functioning immune system, the CMV antigens elicit an inflammatory response that is predominantly posterior (e.g., vitritis, papillitis, CME, and ERM). Treatment may require topical, periocular, or intraocular steroids. Antivirals should be continued to avoid reactivation of CMV in cases of borderline CD4+ counts.
CMV is the most frequent ocular opportunistic infection in patients with AIDS, but is 80% to 90% less common in the era of combination antiretroviral therapy (cART). CMV is almost never seen unless the CD4+ count is <100 cells/mm3. Because active retinitis is often asymptomatic, patients with CD4+ counts <100 cells/mm3 should be seen at least every 3 to 6 months. May also be seen in other immunocompromised states (e.g., leukemia and post-transplant). Local ocular immunosuppression (regional steroid injections) may precipitate CMV retinitis in otherwise healthy patients.