Decreased visual acuity, dyschromatopsia, or visual field defect after a traumatic injury to the eye or periocular area; other trauma symptoms (e.g., pain, periocular edema).
A new afferent pupillary defect in a traumatized orbit that cannot be accounted for by previously existing or concomitant ocular pathology. See Video: Relative Afferent Pupillary Defect.
Decreased color vision in the affected eye, a visual field defect, and other signs of trauma. Acutely, the optic disc appears normal in most cases of posterior indirect TON. In cases of anterior TON, optic disc avulsion may be obvious on funduscopic examination unless obscured by VH. Extraocular motility may be compromised in these cases because of associated EOM avulsion or contusion. TON may be associated with intracranial injury.
Optic disc pallor usually does not appear for weeks after a traumatic optic nerve injury. If pallor is present immediately after trauma, a preexisting optic neuropathy should be suspected. |
Differential Diagnosis of a Traumatic Afferent Pupillary Defect
Severe retinal trauma: Retinal abnormality is evident on examination.
Traumatic, diffuse VH: Obscured view of retina, RAPD is mild if present.
Intracranial trauma with asymmetric damage to the prechiasmal optic nerves.
TON is typically categorized based on location of injury (anterior or posterior) and mechanism of injury (direct or indirect). Anterior TON is arbitrarily defined as occurring anterior to the entrance of the central retinal artery into the optic nerve. Direct TON is usually due to compression, contusion, and/or laceration of the optic nerve. Indirect TON is typically due to deceleration injury with shearing of the nerve and vascular supply in the optic canal, and much less commonly due to rapid rotation of the globe leading to optic nerve head avulsion.
Compressive optic neuropathy from orbital hemorrhage: Most common TON. (See 3.20, Traumatic Retrobulbar Hemorrhage [Orbital Hemorrhage].)
Compressive optic neuropathy from orbital foreign body: A subcategory of direct TON. (See 3.22, Intraorbital Foreign Body.)
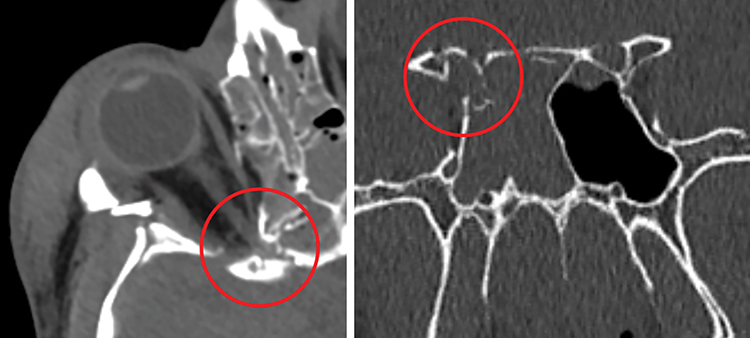
Bony impingement: A posterior direct TON that results from impingement of the apical or intracanalicular optic nerve from a fracture at the orbital apex and/or optic canal. Mechanisms vary widely. Direct bony impingement on the optic canal may result from a skull base fracture that also involves adjacent structures, including the cavernous sinus, with resultant cranial neuropathy (See Figure 3.21.1).
Optic nerve sheath hematoma: An extremely rare and difficult-to-diagnose direct or indirect TON. Imaging may show perineural blood in the optic nerve sheath. Often a presumptive diagnosis requiring an abnormal fundus appearance, typically a combination of retinal venous and arterial occlusions (e.g., central retinal artery occlusion, central retinal vein occlusion). Progressive visual loss may occur as the hematoma expands. In most cases, optic nerve sheath hematoma is seen in conjunction with retinal hemorrhages and a subarachnoid intracranial bleed (Terson syndrome).
Deceleration injury: The second most common form of TON, specifically known as posterior indirect TON, but often simply referred to as TON. The skull (usually the forehead, but can be the midface) hits a static object (e.g., steering wheel, bicycle handlebars, pavement) while the soft tissue within the orbit continues to move forward. Since the optic nerve is tethered at the optic canal, shearing of the nutrient pial vessels may occur with subsequent optic nerve edema within the confined space of the optic canal. A second “shock wave” mechanism may also occur. Cadaver studies have shown that a blow to the frontal bone is transmitted to the optic canal. Visual loss from posterior indirect TON is typically immediate and progresses in fewer than 10% of cases.
Others (e.g., optic nerve laceration, prechiasmal optic nerve avulsion).
History: Mechanism of injury (e.g., deceleration, blow to the forehead)? Loss of consciousness, nausea and/or vomiting, headache, clear nasal discharge (suggestive of cerebrospinal fluid leakage)? Past ocular history including history of amblyopia, strabismus surgery, previous optic neuropathy, retinal detachment, glaucoma, etc.?
Complete ocular examination including an assessment of visual acuity and pupils. This may be difficult depending on the patient’s mental status, use of sedatives, narcotics, etc.
Color vision testing in each eye. Checking red desaturation is a useful alternative if Ishihara color plates are not available.
Visual fields by confrontation. Formal visual field testing is helpful if available.
CT scan of the head and orbit (axial, coronal, and parasagittal views) with thin (i.e., 1-mm) sections through the optic canal and skull base to rule out an intraorbital foreign body or bony impingement on the optic nerve. There may be fractures along the cribriform plate, the sphenoid sinus, and the medial wall of the cavernous sinus. A normal CT in no way rules out posterior indirect TON. Similarly, an optic canal fracture does not mean TON is present.
B-scan US if optic nerve head avulsion is suspected but is obscured clinically by a hyphema, VH, or other media opacity.
Compressive optic neuropathy from orbital hemorrhage: See 3.20, Traumatic Retrobulbar Hemorrhage (Orbital Hemorrhage).
Compressive optic neuropathy from orbital foreign body: See 3.22, Intraorbital Foreign Body.
Optic nerve sheath hematoma: Optic nerve sheath fenestration may be helpful in the acute stage if optic neuropathy is progressing and no other cause is evident.
Optic nerve head avulsion: No effective treatment. If external ophthalmoplegia is present, surgical exploration for avulsed EOM may be necessary.
Deceleration injury: Effective treatment of posterior indirect TON is, at best, extremely limited. Given the results of the Corticosteroid Randomization After Significant Head Injury (CRASH) study, high-dose corticosteroids should never be offered by ophthalmologists to patients with concomitant TBI or if the TON is older than 8 hours. In the vast majority of cases, we recommend observation alone. If corticosteroids are considered (no evidence of TBI, injury within 8-hour window, no medical comorbidities), the lack of definitive therapeutic evidence and significant side effects must be discussed with the patient and/or family and the primary care team; as a practical matter, this scenario is not frequently encountered. Dosing of methylprednisolone includes a loading dose of 30 mg/kg and then 5.4 mg/kg q6h for 48 hours. Proton-pump inhibitors (e.g., omeprazole) should be given concomitantly. More recently, erythropoietin and transcorneal electrical stimulation have been studied as potential treatments for TON. To date, the evidence is limited by study design, low study power, and lack of randomization and masking. At present, neither modality should be considered standard of care for TON.
Bone impingement of the optic canal: Endoscopic optic canal and orbital apex decompression may be offered in select cases, especially if the optic neuropathy is progressive. However, this option should be approached with extreme caution because of the proximity to the cavernous sinus and carotid siphon and possible bony instability of the skull base. The procedure should only be performed by an otolaryngologist and/or neurosurgeon experienced in stereotactic endoscopic sinus and skull base surgery. The patient and/or family should also be informed that there are no definitive data that prove the efficacy of this procedure in TON and that optic canal decompression may result in additional damage to the intracanalicular optic nerve. On occasion, a transcranial approach for optic canal decompression is indicated, depending on the location of the bone fragments.
Vision, pupillary reaction, and color vision should be evaluated daily for 1 to 2 days in cases of indirect TON where progression is suspected. With a nonprogressive TON, the patient can follow up in weeks to months to assess for spontaneous improvement. If a secondary etiology is causing a TON, follow-up depends on the intervention offered and may be more frequent and prolonged. If facial and orbital fracture repair is indicated, it is crucial to document the preoperative visual acuity and visual fields (if possible) and to explain to the patient and family that TON is already present in order to avoid later claims of iatrogenic optic nerve injury. In comatose patients with suspected TON who require facial or orbital fracture repair, the family should be informed that only limited assessment of visual function is possible preoperatively, but significant traumatic visual compromise may have occurred. Always remember that a RAPD may indicate asymmetric, bilateral TON; resist any reassurances to the patient’s family of normal contralateral visual function in obtunded or uncooperative patients with a RAPD. Anecdotally, mild-to-moderate posterior indirect TON may show significant spontaneous improvement over 3 to 6 months in 30% to 60% of patients, while severe initial visual loss seems to carry a worse prognosis.