Spinal anesthesia involves administering local anesthetic into the subarachnoid space.
- Anatomy
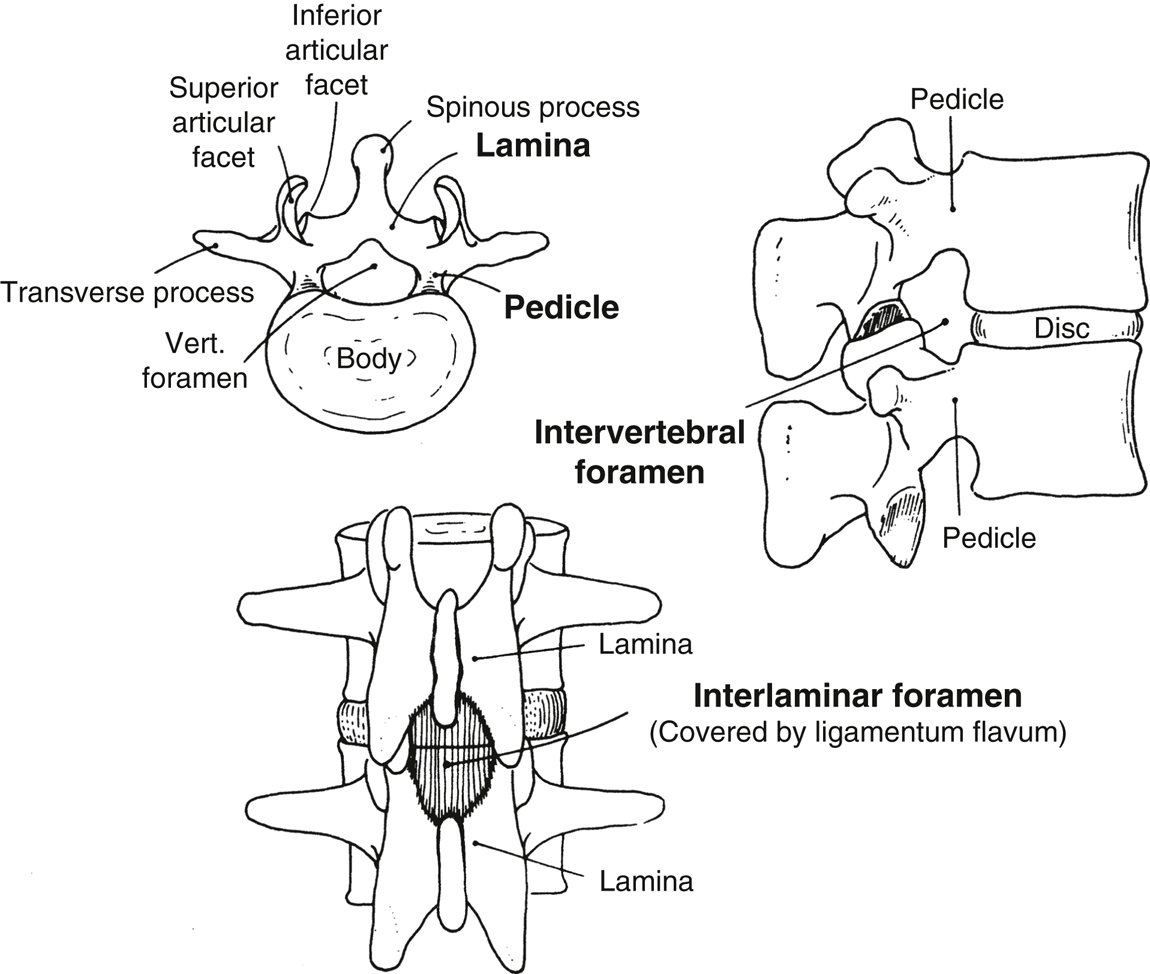
- The spinal canal extends from the foramen magnum to the sacral hiatus. The boundaries of the bony canal are the vertebral body anteriorly, the pedicles laterally, and the spinous processes and laminae posteriorly (Figure 20.2).
- Three interlaminar ligaments bind the vertebral processes together:
- Superficially, the supraspinous ligament connects the apices of the spinous processes.
- The interspinous ligament connects the spinous processes on their horizontal surface.
- The ligamentum flavum connects the caudal edge of the lamina above to the cephalad edge of the lamina below. This ligament is composed of elastic fibers and is usually recognized by its increased resistance to the passage of a needle.
- The spinal cord extends the length of the vertebral canal during fetal life, ends at about L3 at birth, and is moved progressively to a cephalad position as the vertebral column grows to reach near the adult L1 level by 2 years of age. The conus medullaris, lumbar, sacral, and coccygeal nerve roots branch out distally to form the cauda equina. Spinal needles are placed in this area of the canal (below L2) because the mobility of the nerves reduces the danger of trauma from the needle.
- The spinal cord is invested in three meninges:
- The subarachnoid space lies between the pia mater and the arachnoid mater and extends from the attachment of the dura at S2 to the cerebral ventricles above. The space contains the spinal cord, nerves, cerebrospinal fluid (CSF), and blood vessels that supply the spinal cord.
- CSF is a clear colorless fluid that fills the subarachnoid space. The total volume of CSF is 100 to 150 mL, and the volume that exists in the spinal subarachnoid space is ~25 to 35 mL. CSF is continuously formed at a rate of 450 mL/d by secretion or ultrafiltration of plasma from the choroid arterial plexuses located in the lateral, third, and fourth ventricles. CSF is reabsorbed into the bloodstream through the arachnoid villi and granulations that protrude through the dura to lie in contact with the endothelium of the cerebral venous sinuses.
- Physiologic changes
- Neural blockade: Differential blockade refers to the varying sensitivity of different types of nerve fibers to the effects of local anesthetics. Smaller C fibers conveying autonomic impulses are more easily blocked than the larger sensory and motor fibers. As a result, the level of autonomic blockade extends above the level of the sensory blockade by two to six segments. Similarly, fibers conveying sensation are more sensitive to local anesthetics than the larger motor fibers, and as a result, the sensory blockade will extend above the level of the motor blockade. Causes of this phenomenon are multifactorial and include nerve fiber diameter and myelination.
- Cardiovascular: Hypotension is directly proportional to the degree of sympathetic blockade produced. Sympathetic blockade results in dilatation of arteries and venous capacitance vessels, leading to decreased systemic vascular resistance and decreased venous return. If the block is below T4, increased baroreceptor activity produces an increase in activity to the cardiac sympathetic fibers and vasoconstriction of the upper extremities. Blockade above T4 interrupts cardiac accelerator sympathetic fibers, leading to bradycardia, decreased cardiac output, and a further decrease in blood pressure. These changes are more marked in patients who are hypovolemic or elderly or have obstruction to venous return (eg, pregnancy). Risk factors for bradycardia after spinal anesthesia include baseline bradycardia, the American Society of Anesthesiologists physical status 1, use of β-blockers, age younger than 50 years, prolonged PR interval, and sensory level above T6.
- Respiratory: Low spinal anesthesia has no effect on ventilation. With ascending height of the block into the thoracic area, there is progressive ascending intercostal muscle paralysis. This has little effect on ventilation in the supine surgical patient with intact diaphragmatic function mediated by the phrenic nerve (C3 to C5), but patients may report a sensation of dyspnea due to decreased sensation of chest wall expansion. Conversely, ventilation in patients with poor respiratory reserve, such as the morbidly obese, may be profoundly impaired. Paralysis of both intercostal and abdominal muscles decreases the efficiency of coughing, which may be important in patients with chronic obstructive pulmonary disease. Usually, a spinal level of T4 does not result in impaired ventilation, but respiratory compromise may occur in patients with limited respiratory reserve or higher spinal levels.
- Visceral effects
- Bladder: Sacral blockade (S2 to S4) results in an atonic bladder that can retain large volumes of urine. Blockade of sympathetic afferent and efferent innervation of the sphincter and detrusor muscle produces urinary retention.
- Intestine: Sympathetic blockade (T5 to L1) produced by spinal anesthesia leads to contraction of the small and large intestines because of a predominance of parasympathetic tone.
- Neuroendocrine: Peridural block to T5 inhibits part of the neural component of the stress response through its blockade of sympathetic afferents to the adrenal medulla and blockade of sympathetic and somatic pathways mediating pain. Other components of the stress response and central release of humoral factors are unaffected. Vagal afferent fibers from the upper abdominal viscera are not blocked and can stimulate release of hypothalamic and pituitary hormones, such as antidiuretic hormone and adrenocorticotropic hormone. Glucose tolerance and insulin release are normal.
- Thermoregulation: Hypothermia may occur due to several mechanisms. The predominant cause is redistribution of the central heat to the periphery secondary to vasodilatation, which makes forced air warming particularly effective at raising the patient’s temperature. Core temperature may drop even though surface temperature is preserved and patients can feel warm despite a decrease in temperature. Thermoregulation is impaired given the loss of vasoconstriction to preserve heat below the level of sympathectomy. Shivering is common.
- Central nervous system effects: Spinal anesthesia may have direct effects to suppress consciousness, probably secondary to decreased afferent stimulation of the reticular activating system. During spinal or epidural anesthesia, doses of sedative agents may be decreased.
- Technique
- Spinal needle. There are two main categories of spinal needles: those with a beveled tip that cut the dura (“cutting tip”) and those with a conical tip (“pencil-point”) with a lateral opening.
- Sprotte and Whitacre needles: Pencil-point needle; may reduce the incidence of postdural puncture headache (PDPH) (<1%) compared with traditional cutting-tip needles by splitting rather than cutting dural fibers during insertion. Twenty-four and 25-gauge needles are easily bent and are often inserted through a 19-gauge introducer needle.
- Quincke needle: Cutting tip. More prone to causing PDPH. The 22-gauge Quincke needle is more rigid and is easily directed when inserted. It can be useful in older patients in whom access may be more difficult and the incidence of PDPH is low.
- Patient position. The lateral decubitus, prone, and sitting positions can be used for administration of spinal anesthesia.
- In the lateral position, the patient is placed with the affected side up if a hypobaric or isobaric technique is to be used and with the affected side down if a hyperbaric technique is to be used. The spine is horizontal and parallel to the edge of the table. The knees are drawn up toward the chest, and the chin is flexed downward onto the chest to obtain maximal flexion of the spine.
- The sitting position is useful for low spinal blocks required in certain gynecologic and urologic procedures and is commonly used in obese patients to assist in identification of the midline. It is often used in conjunction with hyperbaric anesthetics. The head and shoulders are flexed downward onto the trunk with the arms resting on a stand. An assistant should be available to stabilize the patient, and the patient should not be oversedated.
- The prone position is used in conjunction with hypobaric or isobaric anesthetics for procedures on the rectum, perineum, and anus. A prone jackknife position can be used for administration of spinal anesthesia and the subsequent surgery.
- Procedure
- With all regional anesthetic procedures, the patient should be monitored with standard American Society of Anesthesiologists (ASA) monitors including electrocardiogram (ECG), blood pressure, and oxygen saturation.
- The L2–L3, L3–L4, or L4–L5 interspaces are commonly used for spinal anesthesia. The spinous process of L4 is aligned with the upper borders of the superior iliac crests.
- Disinfect a large area of skin with an appropriate antiseptic solution. Care must be taken to avoid contamination of the spinal kit with antiseptic solution as the solution is potentially neurotoxic.
- Check the stylet for correct fit within the needle; this can be done by pulling the stylet back and then replacing it.
- Raise a skin wheal with 1% lidocaine and a 25-gauge needle at the spinal puncture site.
- Approaches
- Midline. Place the spinal needle (or introducer) through the skin wheal and into the interspinous ligament. The needle should be in the same plane as the spinous processes and angulated slightly cephalad toward the interlaminar space (Figure 20.3).
Figure 20-3 Spinal needle insertion, lateral view.
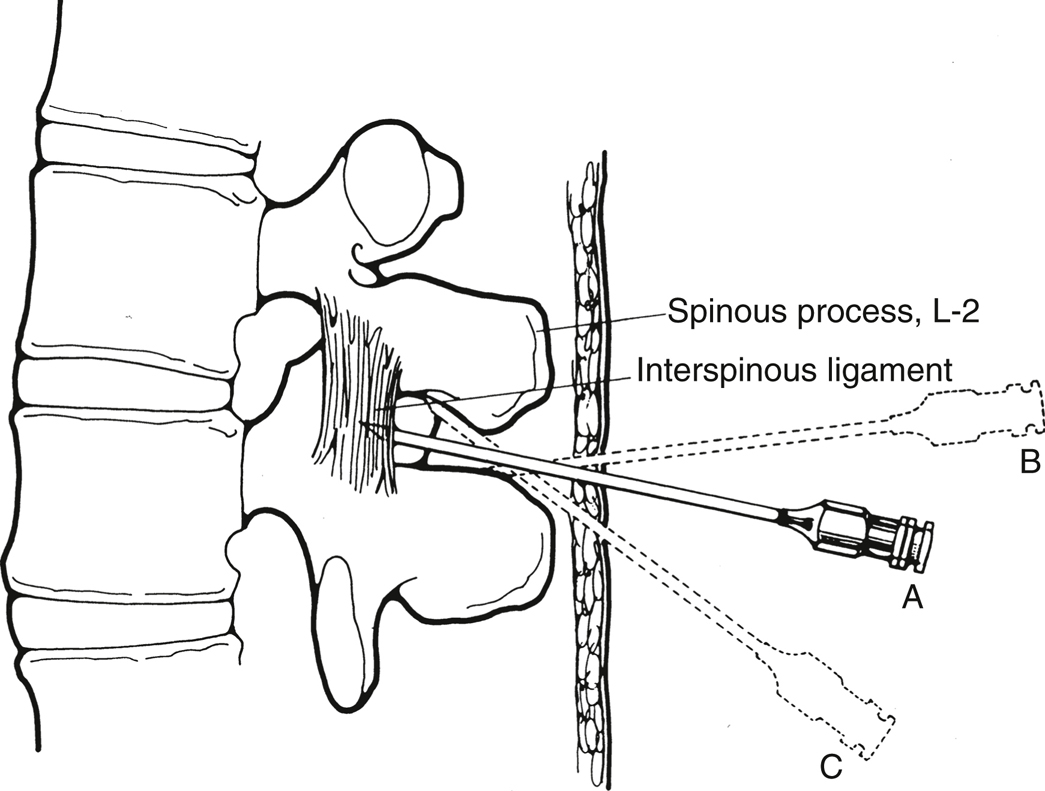
For the classic midline approach, the needle is introduced in the middle of the interspace and advanced with a slight cephalad angulation. If correctly angled (A), it will enter the interspinous ligament, ligamentum flavum, and epidural space. If bone is contacted, it may be the inferior spinous process (B), and cephalad redirection will identify the correct path. If angling cephalad causes contact with bone again at a shallower depth (C), it is probably the superior spinous process. If bone is encountered at the same depth after several attempts at redirection (not shown), the needle is most likely on the lamina lateral to the interspace, and the position of the true midline should be reassessed.
(Reprinted with permission from Mulroy MF. Regional Anesthesia: An Illustrated Procedural Guide. 2nd ed. Little, Brown and Company; 1996:79.)
- Paramedian. This approach is useful in patients who cannot adequately flex their backs because of pain or whose interspinous ligaments may be ossified. Place the spinal needle approximately 1 cm lateral and 1 cm caudad to the center of the selected interspace. Aim the needle medially and slightly cephalad, passing lateral to the supraspinous ligament. If the lamina is contacted, redirect the needle and walk the tip off the lamina in a medial and cephalad direction.
- Needle placement. Always keep the stylet in place when advancing the needle so that the needle’s lumen does not become occluded with tissue. If paresthesias occur during placement, immediately withdraw the needle. Allow the paresthesia to pass and reposition the needle before proceeding again. Advance the needle until increased resistance is felt as it passes through the ligamentum flavum. As the needle is advanced beyond this ligament, a sudden loss of resistance will occur as the needle “pops” through the dura.
- Remove the stylet and confirm correct placement by noting free flow of CSF into the hub of the needle. Rotate the needle in 90-degree increments if necessary to confirm or reestablish good flow of CSF.
- Administration of anesthetic. Connect the syringe containing the predetermined dose of local anesthetic to the needle. Gently aspirate CSF into the syringe, which produces birefringence within dextrose-containing solutions and confirms free flow. Inject the drug slowly. Repeat aspiration of CSF at the end of the injection confirms that the needle point is still within the subarachnoid space. Remove the needle and place the patient gently into the desired position.
- Midline. Place the spinal needle (or introducer) through the skin wheal and into the interspinous ligament. The needle should be in the same plane as the spinous processes and angulated slightly cephalad toward the interlaminar space (Figure 20.3).
- Closely monitor (every 60-90 seconds) blood pressure, pulse, and respiratory function for 10 to 15 minutes. Determine the ascending anesthetic level by noting the response to a gentle pinprick, alcohol swab, or bag of ice. Stabilization of the local anesthetic level takes approximately 20 minutes.
- Continuous spinal anesthesia allows small aliquots of drug to be injected repeatedly to produce the desired level of sensory blockade. With this technique, a high or rapid sympathetic block can be avoided (of particular concern in patients who are sensitive to drastic changes in blood pressure, such as severe aortic stenosis). A 20-gauge catheter is inserted through a 17-gauge epidural needle. The catheter is advanced 2 to 4 cm into the subarachnoid space. Stimulation of nerve roots during catheter insertion necessitates repositioning of the catheter. Neurotoxicity from hyperbaric glucose-containing local anesthetic solutions injected through microbore spinal catheters (26-32 gauge) has been reported and may be due to the development of very high concentrations of local anesthetic around the nerves of the cauda equina. There are currently no such approved microbore catheters marketed in the United States.
- Determinants of the level of spinal blockade
- Major
- Baricity of local anesthetic solution. Local anesthetic solutions can be described as hyperbaric, hypobaric, or isobaric in relation to the specific gravity of CSF (1.004-1.007 g/mL).
- Hyperbaric solutions are typically prepared by mixing the drug with dextrose. They settle by gravity to the most dependent parts of the CSF column (Table 20.2).
Table 20-2 Drugs and Dosages (in mg) for Spinal Anesthesia
Drug Level Duration (minutes) T10 T8 T6 Tetracainea 10 12 14 90-120 Bupivacainea 7.5 9.0 10.5 90-120 Mepivacaine 30 45 60 60-90 - Hypobaric solutions are prepared by mixing the drug with sterile water. They slowly rise to the highest part of the CSF column.
- Isobaric solutions may have the advantage of a predictable spread through the CSF that is less dependent on patient position. Increasing the dose of an isobaric anesthetic has more of an effect on the duration of anesthesia than on the dermatomal spread. Patient positioning can be altered to limit or increase the spread of these mixtures.
- Hyperbaric solutions are typically prepared by mixing the drug with dextrose. They settle by gravity to the most dependent parts of the CSF column (Table 20.2).
- Drug dose. The anesthetic level varies directly with the dose of the agent used.
- Drug volume. The greater the volume of the injected drug, the further the drug will spread within the CSF. This is especially applicable to hyperbaric solutions.
- Patient position. This has a lesser effect on the spread of isobaric solutions.
- Baricity of local anesthetic solution. Local anesthetic solutions can be described as hyperbaric, hypobaric, or isobaric in relation to the specific gravity of CSF (1.004-1.007 g/mL).
- Minor
- Turbulence of CSF. Turbulence created within the CSF during or after the injection will increase the spread of the drug and the level obtained. Turbulence is created by rapid injection, barbotage (the repeated aspiration and reinjection of small amounts of CSF mixed with drug), coughing, and excessive patient movement.
- CSF volume. Lumbosacral CSF volume is inversely correlated with the extent of the spread of local anesthetic.
- Increased intra-abdominal pressure. Pregnancy, obesity, ascites, and abdominal tumors increase pressure within the inferior vena cava. This pressure increases blood volume within the epidural venous plexus, concomitantly reducing the volume of CSF within the vertebral column, which permits greater spread of injected local anesthetic. In obese patients, this effect is potentiated further by increased fat within the epidural space.
- Spinal curvature. Lumbar lordosis and thoracic kyphosis influence the spread of hyperbaric solutions. Drug injected above the L3 level will tend to spread cephalad but will be limited by the thoracic curvature at T4 (Figure 20.4).
- Major
- Determinants of the duration of the spinal blockade
- Drugs and dose. A characteristic duration is specific for each drug (see Chapter 19). The addition of opioids to the injected solution can modify the character of the block (see also Chapter 38). Hydrophilic opioids (eg, morphine) offer analgesia that is slow in onset and long in duration. Undesired side effects are more common, and delayed respiratory depression may occur. If hydrophilic opioids are given intrathecally, patients should be closely observed for at least 24 hours. Lipophilic opioids (eg, fentanyl) have a lower risk of delayed respiratory depression. Onset is fast, and duration is moderate.
- Vasoconstrictors. The addition of epinephrine can prolong the duration of some spinal anesthetics by up to 50%. This effect has not been definitively demonstrated for bupivacaine, although there is evidence that epinephrine prolongs the analgesic effects of low-dose bupivacaine and fentanyl combinations used for labor analgesia. A typical concentration of epinephrine used in spinal anesthetics is 1:400,000 to 1:200,000 (2.5-5 μg/mL).
- Complications and side effects
- Neurologic. Nerve injury is infrequent but can be a serious problem. Several types of nerve injury may occur.
- Direct nerve injury related to needle or catheter placement. Pain during insertion of the catheter or injection of the drug is a warning sign for potential nerve injury resulting from needle or catheter placement and requires repositioning of the needle or catheter. Transient paresthesias reported by the patient typically resolve immediately and are usually without any long-term sequelae.
- Transient neurologic syndrome (TNS) is a spontaneous severe radicular pain that occurs after resolution of the spinal anesthetic and may last for 2 to 7 days. Symptoms include buttock and thigh pain described as achy or burning in quality. TNS is usually responsive to conservative measures, such as nonsteroidal anti-inflammatory drugs and warm compresses. The incidence is highest with lidocaine administration but has also been observed with other local anesthetics, including tetracaine, bupivacaine, and mepivacaine. Obesity, ambulatory surgery, knee arthroscopic surgery, and lithotomy position are additional risk factors.
- Back pain following spinal anesthesia may be related to the relaxation of the ligaments that occurs with anesthesia. There is a similar incidence of back pain following general anesthesia, again likely related to the effects of anesthetic agents and muscle relaxants on the structures of the back. Reassurance should be provided.
- Bloody tap. Puncture of an epidural vein during needle insertion may result in either blood or a mixture of blood and CSF emerging from the spinal needle. If the fluid does not rapidly clear, the needle should be withdrawn and reinserted in a different space.
- Spinal hematoma is a surgical emergency. Overall incidence is around 1/150,000. The signs and symptoms of severe back pain and persistent neurologic deficit usually present within 48 hours. Risk is higher among patients who are coagulopathic or anticoagulated. Bloody taps are not generally thought to cause a spinal hematoma in patients with normal coagulation. A bloody tap may be a risk factor for spinal hematoma in patients who undergo subsequent anticoagulation, but there are no data to support the mandatory cancellation of a case under these circumstances. Instead, we advocate direct communication with the surgeon and that a specific risk-benefit decision about proceeding be made on an individual basis. Close postoperative monitoring for signs consistent with hematoma is warranted. Diagnosis is usually made with magnetic resonance imaging (MRI), and treatment is via emergent hematoma evacuation. Because catheter removal as well as needle placement may cause spinal hematoma, anesthesiologists need to check a patient’s coagulation status and the use of anticoagulants not only at the time of needle placement but also at the time of catheter removal. The approach to the use of anticoagulants, antiplatelet agents, and nonsteroidal anti-inflammatory drugs at Massachusetts General Hospital is listed in Table 20.3.
Table 20-3 Anticoagulation and Epidural Anesthesia/Analgesia Guidelines
Drug (Generic) Common Trade Names Time Interval for Catheter Placement or Neuraxial Procedure After Last Dose Time Interval for Catheter Removal After Most Recent Dose Time Interval to Restart Med After Neuraxial Procedure or Catheter Removal Abciximab ReoPro 48 h 48 h 24 h Argatroban Acova At least 6 h; check for normal PTT or ACT Check for normal PTT or ACT 2 h Cilostazola Pletal 42 h 42 h 5 h Clopidogrelb Plavix 7 d 7 d if single 75 mg dose given 6 h Eptifibatide Integrilin 8 h 8 h 24 h Fondaparinuxc Arixtra 7 d 4 d 6 h Heparin subcutaneously (twice daily) Heparin 4-6 h or assess AC status 4-6 h or assess AC status Can be started immediately Heparin IV Heparin IV: 4-6 h
SC: 12 h and assess AC statusIV: 4-6 h
SC: 12 h and assess AC status1 h Dalteparin (low-dose)d Fragmin (≤5000 U daily) 12 h 12 h 4 h Dalteparin (high-dose)d Fragmin (5000 U twice daily or 120 U/kg twice daily or 175 U/kg daily) 24 h 24 h 4 h Enoxaparin (low-dose)d Lovenox (<60 mg daily) 12 h BID dosing: indwelling catheter should be removed before initiation
Daily dosing: 12 h after last dose4 h after catheter removal and >12 h after needle/catheter placement Enoxaparin (high-dose)d Lovenox (>60 mg daily or 1 mg/kg twice daily) 24 h Indwelling catheter should be removed before initiation 4 h after catheter removal and >24 h after needle/catheter placement NSAID, ASA Celebrex, Motrin, Naprosyn, Vioxx, etc. No significant risk Thrombolytics: streptokinase, alteplase (tPA) Streptase, Activase 10 d 10 d 10 d Ticagrelor Brilinta 5 d 5 d 6 h Tirofiban Aggrastat 8 h 8 h 24 h Warfarin Coumadin 3-5 d, INR ≤ 1.5 Check INR if treatment > 24 h, INR ≤ 1.5 Same day ACT, activated clotting time; ASA, aspirin; NSAID, nonsteroidal anti-inflammatory drug; PTT, partial thromboplastin time.
a If cilostazol (Pletal) is the only anticoagulant given, then an epidural catheter placement is most likely safe. If cilostazol (Pletal) is combined with other anticoagulant medicines (eg, aspirin), neuraxial procedure or catheter placement should be delayed for at least 48 h.
b Clopidogrel (Plavix) has a 24- to 48-h window to remove an epidural catheter once the medication is given. If it has been more than 48 h since dosing of clopidogrel, you must wait for 7 d.
c Fondaparinux should not be given if regional anesthesia is anticipated or has been used. If, however, fondaparinux 2.5 mg is given, we suggest the above guidelines. For larger doses (5-10 mg), neuraxial procedure should not be done for 7 d.
d Low-molecular-weight heparin: single daily dosing may be started 6 to 8 h postoperatively. Twice-daily dosing should be started at least 24 h postoperatively. Epidural catheters should be removed before initiation of therapy.
- PDPH usually develops within 3 days; 70% of headaches resolve within 7 days and 90% within 6 months. The classic “spinal headache” is frontal and occipital in distribution, although the temporal area can be affected as well. The headache is exacerbated by upright posture and relieved by lying supine. Other manifestations can include visual disturbances or hearing impairment. Risk factors include younger age, female gender, larger needle size, pregnancy, multiple dural punctures, and a history of previous PDPH. The incidence may be reduced by using smaller needles and noncutting needles (eg, pencil-point needles). Initial treatments of symptoms include rehydration, maintenance of supine position, pain medication including opioids, and caffeine. Maintenance of supine position as a prevention is neither proven nor recommended. Caffeine exerts its effect by vasoconstriction of cerebral vessels. The recommended dose of caffeine is 300 to 500 mg orally or IV. One cup of coffee contains 50 to 100 mg of caffeine. If initial therapy fails and severe symptoms persist more than 24 hours, an epidural blood patch can be done. Epidural needle insertion is done at the level of presumed dural puncture. Blood is drawn in a sterile fashion and injected into the epidural space. This procedure usually requires two providers. The typical blood volume is 20 to 30 mL or less if the patient complains of back discomfort during injection. The success rate ranges from 65% to 98%, and results are often immediate. A second blood patch can be tried with a success rate about the same as the first attempt. The use of a prophylactic epidural blood patch before the onset of headache symptoms is of controversial benefit, but is still performed by some anesthetists.
- Cardiovascular
- Hypotension. The incidence of hypotension may be reduced by IV administration of 500 to 1000 mL of isotonic solution before performing the block. Patients with decreased cardiac function require care in administering large volumes of IV fluid, because translocation of fluid from the peripheral to the central circulation during cessation of the block and return of systemic vascular tone could produce volume overload and pulmonary edema. Treatment of hypotension includes increasing venous return and treating severe bradycardia. Trendelenburg position, fluid administration, raising lower extremities to autotransfuse blood, or the use of vasopressors may be necessary.
- Bradycardia. Bradycardia can be a sign of a vagal response or could be indicative of a high spinal (less common). If present, bradycardia can be treated with atropine or glycopyrrolate. If bradycardia is severe and accompanied by hypotension, ephedrine or epinephrine may be used.
- Respiratory
- Dyspnea is a common complaint with high spinal levels. It is caused by the proprioceptive blockade of afferent fibers from abdominal and chest wall muscles. Reassuring the patient may be all that is required, although adequate ventilation must be ensured. Supplemental O2 can be provided for patient comfort.
- Apnea can be caused by reduced medullary blood flow accompanying severe hypotension or from direct blockade of C3 to C5 (“total spinal”), inhibiting phrenic nerve output. Immediate ventilatory support is required in this scenario, so the provider should be prepared to mask ventilate and secure the airway if indicated.
- Visceral
- Urinary retention. The mechanism of urinary retention is described in Section IV.B.4.a. Urinary retention may outlast the sensory and motor blockade. A urinary catheter should be placed if anesthesia or analgesia is maintained for a prolonged period.
- Nausea and vomiting are usually caused by hypotension or unopposed vagal stimulation. Treatment involves first restoring blood pressure, followed by administering oxygen, and IV atropine if needed.
- Infection after spinal anesthesia is exceedingly rare. Nevertheless, meningitis, arachnoiditis, and epidural abscess can occur. Possible etiologies include chemical contamination and viral or bacterial infection due to lack of sterility. Consultation and prompt diagnosis and treatment are essential.
- Pruritus commonly occurs with the use of neuraxial opioids and is more frequent with intrathecal administration as opposed to epidural. The exact mechanism is unclear. Pharmacologic treatments include nalbuphine (5-10 mg IV), naloxone (1-2 μg/kg/h), naltrexone (6-9 mg PO), diphenhydramine (25-50 mg IV/PO), ondansetron (4-8 mg IV), and propofol (10-20 mg IV bolus).
- Shivering has a high incidence and may be treated with IV meperidine (25 mg IV). Clonidine (65-300 μg IV) has been shown to have a similar efficacy.
- Neurologic. Nerve injury is infrequent but can be a serious problem. Several types of nerve injury may occur.