- Nonopioid pharmacotherapeutic agents can be used as sole or adjuvant therapy for the treatment of pain. Common medications include the following:
- Acetaminophen 10 to 15 mg/kg PO (IV administration is also 10-15 mg/kg) and 30 to 45 mg PR. Daily dosing should not exceed 75 mg/kg for children, 60 mg/kg for neonates, and 45 mg/kg for preterm infants.
- Ketorolac given 0.5 mg/kg IV or 1 mg/kg IM (given q6h). Caution is used in patients less than 2 years old.
- Additional agents include ketamine, gabapentin, dexmedetomidine, clonidine, and magnesium.
- Opioid therapy is summarized in Table 33.8.There are multiple PO and IV agents to achieve analgesia. Codeine received a black box warning by the FDA in 2013 and warning in 2012 for tonsillectomy and adenoidectomy and caution in postsurgical pediatric for rare but adverse events (rapid metabolism to morphine), respectively. Controversy exists surrounding the use of remifentanil and the induction of rapid tolerance and hyperalgesia. It should also be noted that clearance is higher in neonatal patients. As previously discussed, remifentanil can be used to facilitate endotracheal intubation; caution should be taken due to the side effects of hypotension and bradycardia, especially with elevated dosing.
Table 33-8 Guidelines for Opioid Administration in Pediatric Patients
Drug Starting IV Dose and Interval Parenteral: Oral Dose Ratio Starting Oral Dose and Interval Codeine – – 0.5-1 mg/kg q 3-4 h Morphine 0.05-0.1 mg/kg q2-4 h 1:3 0.3 mg/kg q 3-4 h Oxycodone – – 0.1-0.2 mg/kg every 3-4 h Methadone 0.1 mg/kg every 4-8 h 1:2 0.1 mg/kg every 4-8 h Fentanyl 0.5-1.0 μg/kg every 1-2 h
Infusion: 0.5-2.0 μg/kg/h– – Hydromorphone 0.02 mg/kg every 2-4 h 1:4 0.04-0.08 mg/kg every 3-4 h Meperidine 0.8-1.0 mg/kg every 2-3 h 1:4 2-3 mg/kg every 3-4 h Remifentanil 0.1-0.25 μg/kg
1-4 μg/kg for intubation
Infusion 0.05-0.15 μg/kg/min– – - Regional and neuraxial anesthesia for pediatric patients has gained acceptance because of a better understanding of the pharmacokinetics and pharmacodynamics of local anesthetics in infants and children and the availability of specifically designed equipment. In addition, regional anesthesia has been demonstrated to be safe when performed during general anesthesia, and complication rates are comparable under general anesthesia versus sedated or awake children.
- Pharmacology of local anesthetics
- Protein binding of local anesthetics is decreased in neonates because of decreased levels of serum albumin. Free drug concentration may be increased, especially for bupivacaine.
- Plasma cholinesterase activity may be decreased in infants less than 6 months old, which theoretically diminishes clearance of amino esters.
- Hepatic microsomal enzyme systems are immature in the neonate, and this will decrease the clearance of amino amides.
- The increased volume of distribution in the infant and child acts to decrease free local anesthetic concentrations in the blood.
- Systemic toxicity is the most frequent complication of regional anesthetics, and doses should be carefully calculated on a weight basis. The risk of accumulation of free drug after repeated doses of local anesthetics is increased in infants and children.
- Brachial plexus block and additional regional blocks.
- Brachial plexus blocks (for upper extremity surgery), penile blocks (for circumcision), and ilioinguinal blocks (for inguinal herniorrhaphy) are particularly useful and common regional techniques in the pediatric population. In addition, regional techniques as performed in pediatric patients as in the adult population have yielded great postoperative analgesia and are discussed in Chapter 18.
- Spinal anesthesia
- Indications
- Premature infants less than 60 weeks postconceptual age and infants with a history of apnea and bradycardia, BPD, or need for long-term ventilatory support are at increased risk for apnea and cardiovascular instability after general anesthesia. Spinal anesthesia may decrease the likelihood of these postoperative anesthetic complications. The benefit of spinal anesthesia is beyond postoperative pain management. These techniques in infants can avoid airway instrumentation, decrease the risk of early postoperative apnea, and maintain hemodynamic stability. In the Cochrane review done by Jones LJ et al, it was demonstrated that moderate-quality evidence supports spinal anesthesia is preferred comparing to general anesthesia with possible risk of postoperative apnea reduced up to 47% in preterm infants. The number needed to treat is estimated at 4. In the recently published study done by Liu et al, it is demonstrated that infants that received spinal anesthesia for hernia repair have significantly lower r-FLACC score (the revised Face, Legs, Activity, Cry, and Consolability score) and less requirement for acetaminophen postoperatively in infants younger than 60 weeks postmenstrual age comparing to general anesthesia. These infants still require a minimum of 24 hours of cardiorespiratory monitoring postoperatively, regardless of the anesthetic technique. IV or inhalational medications during spinal anesthesia may negate the potential benefits of protection toward early postoperative apnea.
- Children at risk for malignant hyperthermia.
- Children with chronic airways disease such as reactive airway disease or cystic fibrosis.
- Cooperative older children and adolescents with full stomachs undergoing peripheral emergency surgery (eg, fractured ankle).
- Anatomy. The spinal cord in an infant terminates at approximately L3 and does assume the adult L1-L2 termination until approximately 12 months.
- Technique
- The procedure may be performed with the patient in the lateral decubitus or sitting position in the OR using a team approach. All infants undergoing spinal anesthesia had EMLA cream applied to the L4-L5 interspace and covered with a Tegaderm for 30 minutes before coming into operation room. An assistant is present for positioning the infant (refer to Figure 33.1). Premature infants and neonates are positioned in the sitting position to limit rostral spread of drug. The head is supported upright to prevent upper airway obstruction. A 22-gauge 1.5-inch spinal needle is used for infants, because CSF flow can be very slow especially with a smaller needle. In children older than 2 years, a 25-gauge needle is acceptable. A timer is started as soon as the spinal is placed. We place IV catheter in one of the extremities following the placement of spinal anesthesia. Absence of infant response to attempted IV placement in the lower extremity or detection of lower extremity motor paralysis was used as an indication for successful onset of spinal anesthesia. If an IV was unable to be placed in the lower extremities, it was then attempted in the upper extremities. The surgeons are informed of the time since spinal placement at 30, 45, and 60 minutes to help track progression of the surgery.
Figure 33-1 Demonstration of spinal anesthesia for infant surgeries.
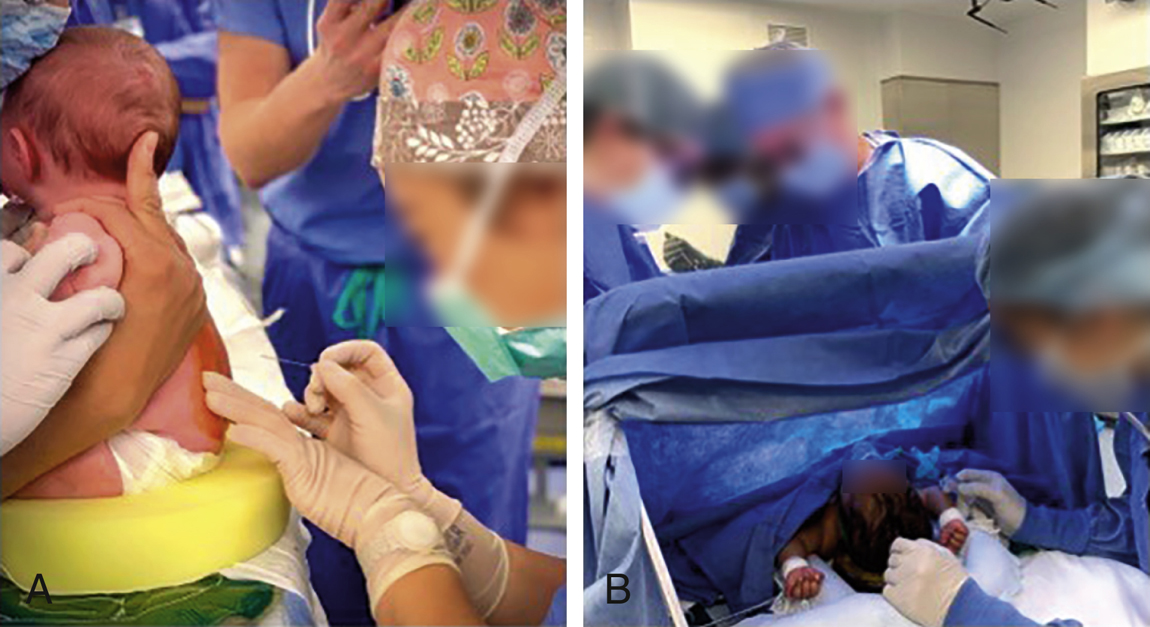
A, Optimized patient position for spinal anesthesia is achieved using a team approach.
B, An ether screen was placed so that the awake patient could be monitored, soothed, and reached easily during surgery. It is crucial to position infants in a way so that they are ready for induction of general anesthesia and intubation at any time during the spinal.
- Patient should be monitored throughout the procedure. Maintaining normothermia is essential, especially for premature infants and neonates. The infant should remain supine after placement of the spinal anesthetic; the Trendelenburg position and leg raising should be avoided because of possible cephalad migration in the subarachnoid space. Special attention should be paid to the tone of crying in the infants under spinal anesthesia. Muffled or weaken crying is a sign of high spinal in this population. Rarely, transient Horner syndrome has been reported in this population with no report for long-term effect so far.
- The procedure may be performed with the patient in the lateral decubitus or sitting position in the OR using a team approach. All infants undergoing spinal anesthesia had EMLA cream applied to the L4-L5 interspace and covered with a Tegaderm for 30 minutes before coming into operation room. An assistant is present for positioning the infant (refer to Figure 33.1). Premature infants and neonates are positioned in the sitting position to limit rostral spread of drug. The head is supported upright to prevent upper airway obstruction. A 22-gauge 1.5-inch spinal needle is used for infants, because CSF flow can be very slow especially with a smaller needle. In children older than 2 years, a 25-gauge needle is acceptable. A timer is started as soon as the spinal is placed. We place IV catheter in one of the extremities following the placement of spinal anesthesia. Absence of infant response to attempted IV placement in the lower extremity or detection of lower extremity motor paralysis was used as an indication for successful onset of spinal anesthesia. If an IV was unable to be placed in the lower extremities, it was then attempted in the upper extremities. The surgeons are informed of the time since spinal placement at 30, 45, and 60 minutes to help track progression of the surgery.
- Drugs and dosage
- Hyperbaric solutions of bupivacaine or tetracaine are used most frequently.
- The dosage requirements are increased, and the duration of action is decreased in infants.
- Recommended dosages for infants, for a T6 level of anesthesia.
- Bupivacaine 0.5% (isobaric solution): 0.5 to 1 mg/kg.
- Bupivacaine 0.75% in 8.25% dextrose: 0.5 to 1 mg/kg.
- Tetracaine, 1% in 5% dextrose: 0.8 to 1.0 mg/kg in an infant and 0.25 to 0.5 mg/kg in a child. This dose is large compared with adult dosage, but it is necessary in infants.
- Duration of surgical anesthesia averages 90 minutes with both tetracaine and bupivacaine. Multiple agents have been added to the local anesthetic in attempts to prolong the block. Epinephrine 2 to 5 μg/kg and clonidine 1 μg/kg have been shown to prolong duration of spinal block.
- Complications and contraindications
- The anesthetic level recedes more quickly in children than in adults. If the block subsides, supplemental sedation must be used cautiously, especially in premature infants and neonates. If subarachnoid anesthesia is inadequate, it is best to initiate general anesthesia before positioning.
- Hypotension is rare in children less than 7 to 10 years old, perhaps because resting sympathetic vascular tone is lower than in adults. A high spinal anesthetic may be heralded only by mottled skin or apnea and bradycardia.
- Contraindications are similar to those in adults, with particular attention to congenital anatomic defects of the central nervous system and a history of intraventricular hemorrhage.
- Indications
- Caudal and epidural anesthesia
- Indications. These techniques are useful in combination with general anesthesia for minor and major procedures of the thorax, abdomen, pelvis, bladder, and lower extremities, particularly when significant postoperative pain is anticipated (eg, orthopedic surgery).
- Anatomy is outlined in Chapter 17. Note that the dural sac ends at the level of the S3 vertebra in the neonate; care is required to avoid dural puncture during placement of the caudal needle.
- Technique is outlined in Chapter 17.
- Most caudal and lumbar epidural anesthetics are placed after induction of general anesthesia.
- Caudal anesthesia may be administered as a single injection of local anesthetic through a 1.5-inch, short-bevel styletted needle placed into the caudal epidural space. This technique is ideally suited for short procedures with mild-to-moderate postoperative pain such as inguinal herniorrhaphy, orchiopexy, and circumcision. For longer procedures or prolonged postoperative analgesia, a catheter may be advanced from the sacral epidural space. Intermittent boluses or a continuous infusion of local anesthetic with or without an opioid may be used. In infants, 22-gauge caudal catheters are placed through 20-gauge, 40- to 50-mm Tuohy needles; older children require 20-gauge catheters placed through 17- or 18-gauge, 90- to 100-mm Tuohy needles.
- Caudal catheters can be advanced to lumbar or thoracic levels in young children because the epidural space is not yet extensively vascularized. The recommended levels are T6-T9 vertebral level for thoracic surgery (eg, pectus excavatum repair), T10-T12 vertebral level for abdominal surgery (eg, Nissen fundoplication or bowel resections), and L3-L4 vertebral level for pelvic procedures. Usually, these catheters advance easily; resistance may indicate malpositioning. If necessary, confirmation of catheter placement can be done with contrast dye, stimulation, ECG, and fluoroscopy techniques. While easy to place when compared with a lumbar catheter, the caudal catheter has a greater potential to become contaminated from stool. Also, the catheter may become dislodged postoperatively.
- Epidural catheters may be placed via lumbar or thoracic approaches. The distance from the skin to the epidural space is short (1-2 cm) in children, and, again, care must be taken to avoid dural puncture. Loss of resistance is usually accomplished with saline. In older children, 18-gauge Tuohy needles and 20-gauge catheters are used. Thoracic catheters are useful for petus excavation repair or thoracotomy.
- Drugs and doses
- In single-dose caudal anesthesia, a long duration of sensory blockade with minimal motor blockade is desirable. Bupivacaine, 0.125% to 0.25% with epinephrine, is administered according to the formula of 0.06 mL of local anesthetic per kg per segment, where the number of segments is counted from the S5 spinal level to the desired level of analgesia. A simple alternative dosing scheme is to administer 0.125% bupivacaine with epinephrine at a dose of 1 to 1.25 mL/kg. Increasing the concentration of bupivacaine above 0.25% does not appear to improve analgesia. Dosages of bupivacaine 2.5 mg/kg without epinephrine and 3 mg/kg with epinephrine result in plasma levels in infants and children below the toxic range determined for adults. Ropivacaine 2% has been successfully used in caudal anesthesia at doses of 1 mL/kg for minor elective surgery. The addition of clonidine 0.5 to 2 μg/kg to bupivacaine prolongs the duration of analgesia by 2 to 3 hours. It may cause increased sedation postoperatively and should be avoided in babies at risk of apnea (neonates and ex-preterm infants).
- Continuous infusion: Bupivacaine 0.05% to 0.1% or ropivacaine 0.2% can be infused epidurally at a dose of 0.2 to 0.3 mg/kg/h in infants and 0.2 to 0.4 mg/kg/h in children. Opioids may be added in μg to the local anesthetic solution: fentanyl (1-3 μg/mL), infused at 0.3 to 1 μg/kg/h; morphine 5 to 10 μg/mL, infused at 1 to 5 μg/kg/h; or hydromorphone 3 to 7 μg/mL, infused at 1 to 2.5 μg/kg/h. Infants younger than 6 to 12 months generally do not receive opioids in the epidural infusion, unless they are in a closely monitored setting.
- Postoperative analgesia may be provided by infusion through the caudal or epidural catheter. Generally, an infusion of 0.1% bupivacaine with fentanyl, 1 to 3 μg/mL at 0.3 to 1 μg/kg/h, will provide good analgesia without motor blockade. However, some patients benefit from omission of local anesthetic from the infusion, and fentanyl, 0.5 to 1 μg/kg/h, can be used in these patients. Because of concern about postoperative respiratory depression, infants younger than 6 to 12 months generally do not receive epidural opioids unless they are in a closely monitored setting. These infants receive an infusion of 0.1% bupivacaine, 0.2 to 0.4 mL/kg/h.
- Contraindications are the same as for spinal anesthesia (see Section XI.F.5).
- Chlorhexidine use in infants less than 2 months old or with skin breakdown is controversial due to systemic absorption and risk of burn injury.
- Complications of epidural and caudal anesthesia are discussed in Chapter 17.