Abdominal aortic repair may be required for atherosclerotic occlusive disease or aneurysmal dilation of the aorta. Aorto-occlusive disease usually presents as claudication. An abdominal aortic aneurysm (AAA) may be found incidentally. Alternatively, it may present with back pain or, if ruptured, severe shock. Ninety-five percent of all AAAs occur below the level of the renal arteries. Indications for repair are largest diameter greater than 5.5 cm; rate of expansion exceeding 1 cm/y; or symptoms secondary to the AAA. In females, repair of asymptomatic AAA may be indicated at a lower diameter threshold due to a higher rate of rupture, although females may also be at a higher perioperative risk. The annual risk of rupture of an expanding 5-cm aneurysm is about 4%. The operative mortality for elective AAA repair is less than 2%, while the overall mortality of aneurysm rupture is 70% to 80%.
- Endovascular abdominal aortic repair (EVAR)
- Overview. The majority of infrarenal AAA are treated by EVAR. The procedure involves the endovascular placement of an expandable stent graft spanning the aneurysm under fluoroscopic guidance. The stent graft excludes the aneurysm from the circulation preventing its expansion and rupture. The application of an aortic cross clamp is not required consequently. EVAR leads to greater intraoperative hemodynamic stability. There is a lower incidence of postoperative pulmonary, cardiovascular, and renal morbidity and significantly reduced 30-day operative mortality for EVAR compared to open surgery. The early survival benefit is lost over time. At about 2 years postprocedure, mortality rates are the same as those for patients who have undergone open aneurysm resection. Patients with endovascular repair also require regular follow-up imaging and may require additional EVAR-related interventions.
- Patient suitability for EVAR depends on the size and morphology of the AAA. Most infrarenal AAAs with favorable neck anatomy are repaired endovascularly. EVAR has lower short-term, perioperative mortality, but higher incidence of leak and other long-term complications, and requires a lifetime of imaging surveillance. Younger patients with better risk profile may more suited for open repair.
- Monitoring and access. Intra-arterial blood pressure monitoring is required in addition to standard monitors. Large bore peripheral access (16 gauge or 14 gauge) is desirable. Central venous access is rarely required.
- Surgical considerations. Systemic heparinization is required. If the aneurysm involves the renal or mesenteric arteries, a more complex endovascular stent graft with branches or fenestrations can be used. For infrarenal aneurysms, surgical access is usually via bilateral common femoral arteries. Exposure of the arteries followed by arteriotomies is typically performed with surgical repair afterward. Percutaneous access with subsequent closure using a specialized device is possible for select patients in the absence of femoral artery disease or stenosis. There is significant radiation exposure with fluoroscopy and a contrast dye load. Ensuring the patient does not move during digital subtraction angiography will reduce the patient’s exposure by minimizing the need for repeated studies. Following stent graft deployment, an intra-aortic balloon is inflated to expand the stent graft and anchor it in place. Balloon inflation leads to temporary aortic occlusion. The occlusion is short lived, typically lasting 3 to 4 heartbeats. Hypertension may be observed during the period of balloon occlusion; however, it usually does not last long enough to warrant therapy. Controlled hypotension may be requested by the surgeon during stent graft deployment to prevent proximal hypertension and stent graft displacement.
- Anesthetic technique. EVAR may be performed under local, regional, or general anesthesia. Factors such as anticoagulant use, patient comfort, surgeon preference, and anticipated length of surgery will influence the choice of anesthetic. There is some evidence to suggest lower morbidity and mortality, shorter hospital stays, and fewer ICU admissions with local or regional anesthesia as compared to general anesthesia. Bilateral ilioinguinal or iliohypogastric nerve blocks are alternatives along with local infiltration. Of note, in contrast to neuraxial anesthesia, local anesthesia will not alleviate the ischemia pain associated with femoral artery occlusion, and supplementation with systemic analgesics may be required.
- Conversion to open repair is rare (<1%). It may be necessary in the setting of difficult arterial access (eg, with severely atherosclerotic arteries); vessel dissection at the arterial access site; tortuous iliac arteries that prevent passage of the deployment system; stent malposition or migration; and aneurysm rupture. Resuscitation equipment such as a red blood cell salvage device and a rapid infusion device should be available in centers performing EVAR.
- Bleeding may occur around the femoral sheaths and is often difficult to quantify or track. Massive hemorrhage is rare.
- Complications of EVAR include failure to exclude the AAA from the arterial system (endoleak), embolism, arterial injury, graft kinking, groin access injury, limb ischemia and infection. AKI can result from contrast administration and embolization of debris dislodged by catheters and wires (see Section I.C).
Endoleak (Figure 28.1) is the persistence of blood flow into the aneurysm sac leading to expansion. It occurs in 20% to 25% of EVARs. Types I and III endoleaks are considered at high risk for aneurysm sac pressurization and rupture and should be treated as soon as diagnosed. Type II endoleaks may spontaneously resolve and are followed closely with serial imaging. If they persist, they may require a subsequent procedure to embolize the responsible vessel. Treatment is not recommended for type IV endoleaks.
Figure 28-1 A and B, Schematic demonstrating the different types of endoleak after endovascular aneurysm repair.
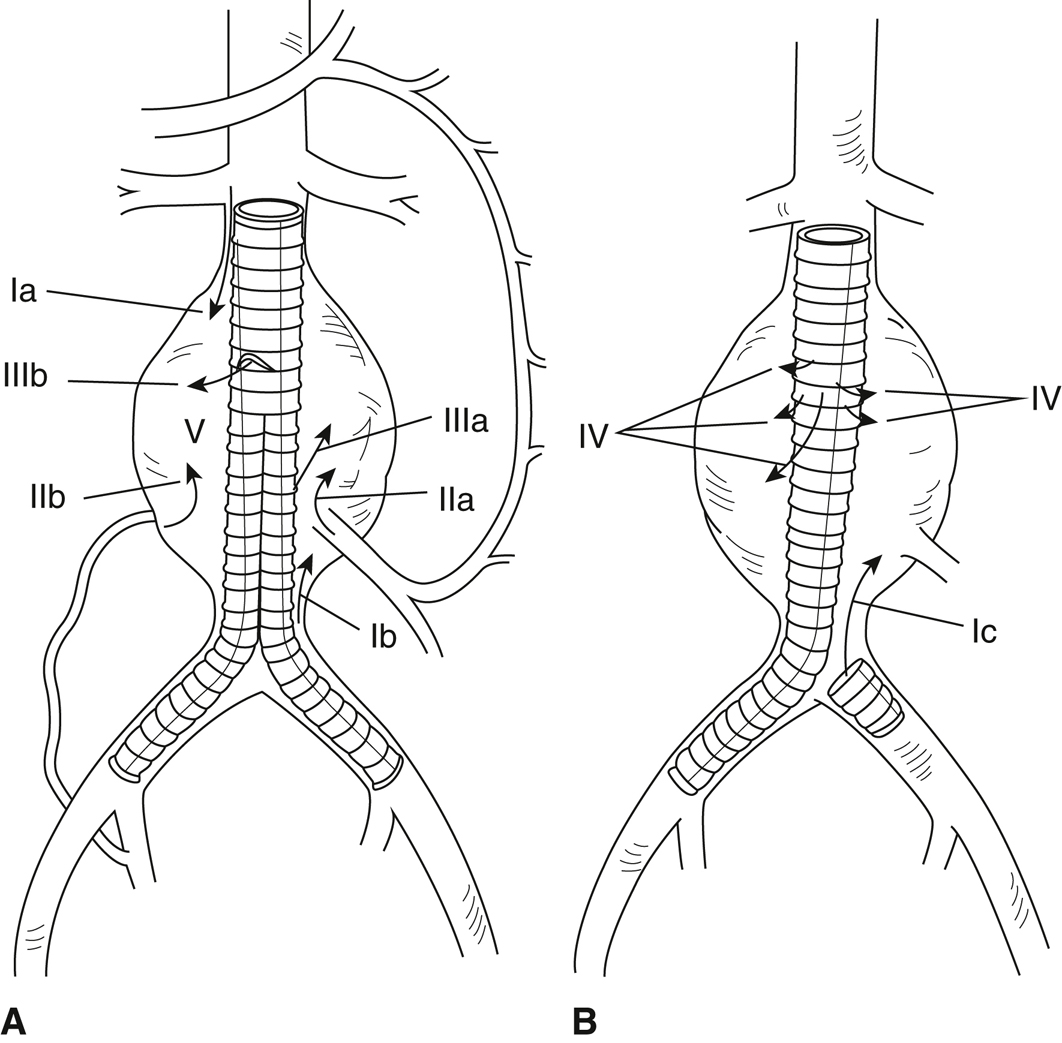
Type I endoleaks occur because of inadequate graft seal resulting in perigraft flow and include (Ia) perigraft flow occurring proximally, (Ib) perigraft flow occurring distally, and (Ic) perigraft flow around an iliac artery occlusion device. Type II endoleaks occur when branch arteries backbleed because of collateral flow. These endoleaks include (IIa) backbleeding inferior mesenteric artery and (IIb) backbleeding lumbar artery. Type III endoleaks occur when flow persists between the segments of a modular graft and include (IIIa) leaks between iliac limbs or an iliac limb and main body component and (IIIb) leaks between main body components. Type IV endoleaks (IV) occur when flow is present through endograft material (graft porosity). Type V endoleak, or, “endotension” (V), occurs when persistent or recurrent pressurization of the aortic aneurysm exists in the absence of demonstrable endoleak.
(Drawings prepared by H. Fischer, MFA.)
- Open abdominal aortic surgery
Patient selection. Open surgical repair is indicated for patients whose aortic anatomy does not meet the specified minimum requirements for standard, commercially available endografts; for patients in whom delaying repair to wait for a custom-made endograft may result in significant risk of rupture; and for patients unlikely to comply with long-term imaging surveillance protocols following EVAR.
- Surgical approach. Compared with a transabdominal approach, the retroperitoneal (RP) approach may result in a lower incidence of postoperative ileus, pulmonary complications, cardiovascular complications, and fluid shifts. The RP approach is technically advantageous in morbidly obese patients and in those who have had previous abdominal procedures.
- Monitoring. A large peripheral IV, arterial line, central venous catheter, and Foley catheter are indicated. Procedures that require a supraceliac cross-clamp entail greater hemodynamic perturbations and blood loss. Enhanced intraoperative monitoring of cardiac function with TEE or PA catheter may be beneficial. Vasopressors and vasodilators must be available.
- Anesthetic technique. Most patients receive combined general and epidural anesthesia using a low-thoracic to midthoracic epidural catheter. Although general anesthesia alone is acceptable, a combined technique reduces anesthetic requirements, facilitates earlier extubation, and provides effective postoperative analgesia. Retrospective data suggests a mortality benefit with epidural use in elective AAA repair.
- The hemodynamic goal of induction is to avoid hypertension. It is helpful to establish a sensory level with the epidural catheter prior to the induction of general anesthesia.
- Heat conservation. Heat loss during aortic procedures may be considerable. Strategies for heat conservation are discussed in Chapter 19. Forced air warmers should never be used below the level of the aortic cross-clamp. Severe burns may develop during cross-clamping due to the absence of heat redistribution by normal blood flow.
- Bowel manipulation is necessary to gain access to the aorta during the transabdominal approach and may be accompanied by skin flushing, decreased systemic vascular resistance, and profound hypotension. These changes may be caused by the release of prostaglandins and vasoactive peptides from the bowel and last for 20 to 30 minutes. Treatment consists of vasopressor administration and volume expansion.
- Fluid management. There is the potential for significant intravascular volume depletion due to hemorrhage; insensible losses into the bowel and peritoneal cavity; and evaporative losses associated with the laparotomy. Volume resuscitation is guided by the patient’s hemodynamics. Aortic cross-clamp application will give rise to renal insufficiency, even with an infrarenal cross-clamp.
- Blood product administration should be guided by the patient’s laboratory values.
- Aortic cross-clamping
- Systemic heparin is administered several minutes prior to the application of the aortic cross-clamp.
- Hypertension will occur with aortic cross-clamping. The degree of hypertension is a function of the cross-clamp location. A more proximal cross-clamp is associated with a greater increase in blood pressure. Consideration can be given to inducing mild hypotension prior to the application of the cross-clamp. The increased afterload is well tolerated by patients with normal cardiac function, but those with compromised left ventricular function may develop myocardial ischemia and hypotension. Hypotension with the application of the aortic cross-clamp may indicate left ventricular failure secondary to increased afterload or retrograde dissection into the coronary arteries. Cardiac function must be evaluated and supported.
- Avoidance of excessive hypertension is important during aortic cross-clamp to minimize the risk of retrograde aortic dissection.
- Following proximal clamping, vascular control distal to the aneurysm is established. If the vasculature distal to the aneurysm has been surgically exposed, distal control may be achieved via the application of a cross-clamp on the aorta or common iliac arteries distal to the aneurysm. If the distal vasculature is not exposed, endovascular control is achieved. Once the aneurysm sac is opened, a balloon-tipped catheter is advanced distal to the aneurysm. Balloon inflation occludes the vessel and prevents backbleeding, establishing distal control. Significant retrograde hemorrhage may occur during this maneuver.
- There is no compelling evidence to support the use of renal protective adjuncts such as mannitol and fenoldopam. The application of an aortic cross-clamp at any level may lead to decreased renal cortical blood flow and urine output. With suprarenal clamping, renal perfusion is at even greater risk due to longer cross-clamp times and potential for cholesterol embolization. The maintenance of adequate hydration and urine output is paramount.
- Aortic unclamping
- Unclamping will produce immediate hypotension due to a fall in systemic vascular resistance.
- The degree of hypotension is a function of clamp duration and the proximal extent of the clamp.
- Prior to release of the aortic cross-clamp, steps should be taken to optimize the patient’s intravascular volume status and increase vascular tone. Inspired oxygen concentration should be increased to 100%. Minute ventilation may also be increased to induce a mild respiratory alkalosis.
- Following unclamping, reperfusion of the ischemic organs and tissues leads to acidemia and systemic vasodilation. The magnitude of these disturbances is a function of the duration of the aortic cross-clamp and the metabolic activity of the organs that were ischemic. Cross-clamping above the celiac or superior mesenteric artery can produce profound visceral ischemia and acidosis. Sodium bicarbonate may be administered at unclamping to mitigate the effects of acidosis. In the setting of profound hypotension that is unresponsive to fluids or vasopressors, the aortic cross-clamp may be reapplied to maintain adequate perfusion pressure while additional volume and vasopressors are administered.
- Emergence. Most patients with working epidurals and infrarenal or juxtarenal cross-clamps can be extubated at the end of the procedure. Patients with hemodynamic instability, persistent hemorrhage, persistent acidosis, or severe hypothermia (<33 °C) are left intubated. Hypertension, tachycardia, pain, and shivering should be anticipated and treated.
- Emergent repair of a ruptured AAA. Patients may be hemodynamically stable or unstable depending on the degree to which the rupture is contained. Rupture can occur into the peritoneal cavity or the RP space. Some measure of tamponade will temporarily reduce the volume of blood loss.
- The priority for anesthetic management is the establishment of adequate IV access for large volume resuscitation.
- Blood products should be ordered immediately. If type-specific blood and FFP are unavailable, universal donor-type blood (type O negative for women of childbearing age and type O positive for all others) and FFP (type AB) should be obtained, and a blood bank sample should be sent immediately to the lab for cross-matching. Colloid solutions should be ordered. A red blood cell salvage system as well as a rapid transfuser should be immediately available.
- Standard monitors should initially be applied if the patient is unstable, so as not to delay definitive surgical control. The immediate priority is to control bleeding surgically by cross-clamping the aorta through a laparotomy or thoracotomy. Alternatively, the aorta may be occluded endovascularly using a balloon-tipped catheter. Invasive monitors (arterial line, central line, PA catheter) may be placed as time and hemodynamics permit. Clear communication with the surgical team regarding the surgical and anesthetic plans is essential. If the patient’s hemodynamics permit, placement of a central venous catheter is often performed while the patient is awake.
- Hypotensive resuscitation that involves restricting intravascular volume administration to maintain SBP in the 70 to 100 mm Hg range has been recommended. Evidence for this management strategy is borrowed from the trauma literature, and this strategy has not been studied in ruptured aortic syndromes. The physiologic rationale behind this strategy is to limit hypothermic and dilutional coagulopathy while avoiding clot disruption caused by increases in SBP.
- Based on the clinical circumstances, the surgical plan, and the available equipment, it may be preferable to insert an occlusive balloon-tipped catheter into the aorta above the level of the aneurysm prior to the induction of general anesthesia. Such a catheter may be inserted percutaneously using local anesthesia. It can then be used to occlude the aorta in the event of circulatory collapse following anesthetic induction.
- Surgical technique: endovascular versus open
- Open repair
- Induction. In moribund patients, endotracheal intubation should be performed immediately. Hypotensive patients should be preoxygenated and induced expeditiously. Hemodynamics may allow for only small doses of scopolamine, etomidate, ketamine, and a benzodiazepine. Severe hypotension on induction may occur as a result of vasodilation, loss of sympathetic drive, and loss of peritoneal tamponade with abdominal wall relaxation. The patient should be prepped and draped prior to induction and the surgeon ready to make incision if needed.
- Care must be taken to avoid hypertension during induction.
- Once the aorta has been clamped and hemodynamic stability achieved, incremental doses of opioids and supplemental anesthetics may be given as tolerated.
- Blood component therapy should be guided by the laboratory values. In the event of massive hemorrhage, packed red blood cells and FFP should be administered in a ratio of at least 2:1.
- Hypothermia contributes to acidosis, coagulopathy, and myocardial dysfunction and should be treated.
- Renal protective strategies such as aggressive hydration and the maintenance of appropriate perfusion pressure should be instituted. The administration of mannitol and fenoldopam may be considered, although there is no compelling evidence to support their use. Mortality in patients developing renal failure following a ruptured AAA is high.
- Endovascular repair can be considered as an alternative to open repair provided anatomy is suitable, the center is appropriately equipped, and the team is appropriately experienced. The procedure may be performed under local anesthesia. Retrospective studies have shown a survival benefit in patients treated with EVAR, but evidence from prospective randomized trials is lacking.
- Open repair
- Complications and prognosis. Under the best of circumstances, there is a 40% to 50% mortality rate. The incidence of MI, acute renal failure, respiratory failure, and coagulopathy is high.