Human genetics is the scientific study of the variations among people that are determined by the heritable units called genes, and how variations due to genes occur and are transmitted in individuals, families, and populations. While the discipline of genetics had its origins with Gregor Mendel in the mid-nineteenth century, human genetics began in the early twentieth century and remains one of the most vibrant of the biologic sciences. Genomics, on the other hand, had its origins more recently, the term first being coined in 1986, and subsumes the study of the organization, function, and interpretation of all an organism's genetic material. Genomics has largely been stimulated and driven by technologies that enable the sequencing of DNA and the comparative analysis of vast amounts of sequence data. Both fields are transforming our understanding of human biology, medicine, and public health.
Clinicians at one time concerned themselves only with what they could discover by bedside evaluation and laboratory investigation. In the parlance of genetics, the patient's symptoms and signs constitute the phenotype. The means are at hand for defining a person's genotype, the actual information content inscribed in the 2 m of coiled DNA present in each cell of the body-or half that amount in every mature ovum or sperm. Most phenotypic characteristics-and this includes diseases as well as human traits such as personality, adult height, and intelligence-are to some extent determined by the genes. The importance of the genetic contribution varies widely among human phenotypes, and the genes involved in complex traits and common diseases are gradually being identified. Moreover, the importance of interactions between environment and genotype in producing phenotypes cannot be overstated despite the obscurity of the actual mechanisms, such as the contributions of the epigenome and the microbiome.
DNA is composed of four nucleotides-adenine (A), guanine (G), cytodine (C) and thymidine (T)-arranged linearly along one strand, which intertwines in a double helix with a complementary strand such that each A pairs with a T and each G with a C. Each human cell nucleus contains 6.4 billion of these nucleotide pairs. About 2% of nuclear DNA is organized in functional units called genes, and each of the approximately 21,000 human genes is accompanied by various regulatory elements that control when it is active in producing messenger RNA (mRNA) by a process called transcription. In most situations, mRNA is transported from the nucleus to the cytoplasm, where its genetic information is translated into proteins, which perform the functions that ultimately determine phenotype. For example, proteins serve as enzymes that facilitate metabolism and cell synthesis; as DNA binding elements that regulate transcription of other genes; as structural elements of cells and the extracellular matrix; and as receptor molecules for intracellular and intercellular communication. DNA also encodes many small RNA molecules that serve functions still being defined, including regulating gene transcription and interfering with the translational capacity of some mRNAs.
Chromosomes are the vehicles in which the genes are carried from generation to generation. Each chromosome is a complex of protein and nucleic acid in which an unbroken double helix of DNA is coiled and supercoiled into a space many orders of magnitude less than the extended length of the DNA. Within the chromosome there occur highly complicated and integrated processes, including DNA replication, recombination, and transcription. In the nucleus of each somatic cell, humans normally have 46 chromosomes, which are arranged in 23 pairs. One of these pairs, the sex chromosomes X and Y, determines the sex of the individual; females have the pair XX and males the pair XY. The remaining 22 pairs are called autosomes (eFigure 42-1). In addition to these nuclear chromosomes, each mitochondrion-found in varying numbers in the cytoplasm of all cells-contains multiple copies of a small chromosome. This mitochondrial chromosome of 16,569 nucleotides encodes 13 of the proteins for oxidative metabolism and all the transfer RNAs used in translation of proteins within this organelle. Mitochondrial chromosomes are inherited almost entirely from the cytoplasm of the fertilized ovum and are therefore maternal in origin.
eFigure 42-1. Normal Karyotype of a Human Male
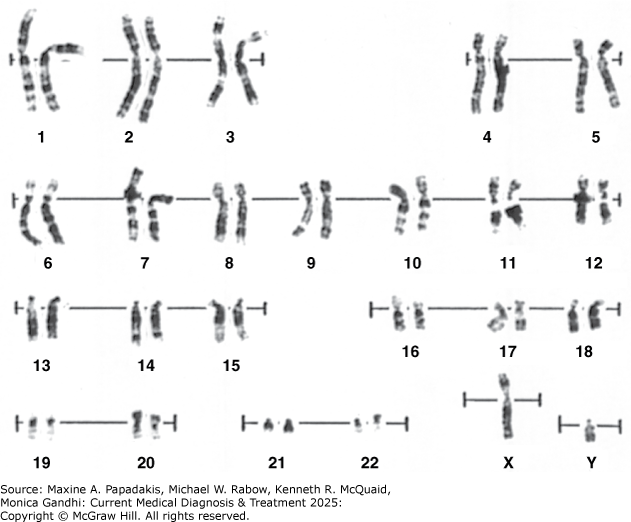
Normal karyotype of a human male. Prepared from cultured amniotic cells and stained with Giemsa stain. About 400 bands are detectable per haploid set of chromosomes.
In all somatic cells, the 44 autosomes and one of the X chromosomes are transcriptionally active. In males, the active X is the only X; portions of the Y chromosome are also active. In females, the requirement for dosage compensation (to be equivalent to the situation in males) is satisfied by inactivation of most of one X chromosome early in embryogenesis. In females, a small portion of the "inactive" X chromosome, the pseudoautosomal region, also is transcriptionally active. This process of X chromosomal inactivation occurs randomly, so that on average, in 50% of a female's cells, one of the X chromosomes will be active, and in the other 50%, the homologous member of the pair will be active. The phenotype of the cell is determined by which genes on the chromosomes are active in producing mRNA at any given time.
Transcriptional control is highly complex. In most cases, both copies of a given gene, termed alleles, are transcribed. However, one allele of some genes is not transcribed into mRNA through various molecular mechanisms including imprinting. Imprinting occurs in the gamete, typically by adding a methyl group to cytosine nucleotides in the regulatory region of the allele. Methylation results in downregulation of the allele and is gamete-specific. Other mechanisms for imprinting involve precise modification of histone molecules that bind to DNA to form what can be visualized as chromosomes. Thus, the allele of some genes inherited from the father is permanently turned off, while the alleles of other genes inherited from the mother are similarly inhibited. Other processes can affect expression of specific alleles, including biochemical modification of certain histones and the spatial organization of chromosomes within the nucleus. Most strikingly, these effects can persist across generations and be influenced by the environment. One example of the latter is the reduction in imprinting seen in times of famine, when methyl groups are deficient in the diet. This field of non-Mendelian inheritance is termed epigenetics.
CookJ. Genes in families. In: PyeritzREet al (editors). Emery and Rimoin's Principles and Practice of Medical Genetics and Genomics: Foundations, 7th ed. Elsevier; 2019:210. MikhailFM. Chromosomal basis of inheritance. In: PyeritzREet al (editors). Emery and Rimoin's Principles and Practice of Medical Genetics and Genomics: Foundations, 7th ed. Elsevier; 2019:237. SadikovicBet al. Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet Med. 2021;23:1065. [PMID: 33547396] |