Key Clinical Updates in Choosing an Antiretroviral Treatment Regimen Demonstration projects are examining long-acting cabotegravir and rilpivirine as injectables in people challenged by adherence or viremi, with high virologic suppression achieved in one demonstration project in San Francisco, with 97.5% virologic suppression projected. Gandhi M et al. Ann Intern Med. [PMID: 37399555] |
Treatment for HIV infection can be broadly divided into the following categories: (1) prophylaxis for opportunistic infections, malignancies, and other complications of HIV infection; (2) treatment of opportunistic infections, malignancies, and other complications of HIV infection; and (3) treatment of the HIV infection itself with ART.
A. Prophylaxis for Complications of HIV Infection
In general, decisions about prophylaxis of opportunistic infections are based on the CD4 count, recent HIV viral load, and a history of having had the infection in the past. Studies have shown that in patients with robust improvements in immune function-as measured by increases in CD4 counts above the levels that are used to initiate treatment-or prolonged virologic suppression in the setting of poor immunologic response, prophylactic regimens can safely be discontinued.
Because people with advanced HIV infection are susceptible to a number of opportunistic pathogens, the use of agents with activity against more than one pathogen is preferable. It has been shown, for example, that trimethoprim-sulfamethoxazole confers protection against toxoplasmosis in individuals receiving this medication for Pneumocystis prophylaxis.
1. Prophylaxis Against Pneumocystis Pneumonia
Patients with CD4 counts below 200 cells/mcL, a CD4 lymphocyte percentage below 14%, or oral candidiasis should be offered primary prophylaxis for Pneumocystis pneumonia. Patients with a history of Pneumocystis pneumonia should receive secondary prophylaxis until their viral load is undetectable and they have maintained a CD4 count of 200 cells/mcL or more while receiving ART for longer than 3 months. Regimens for Pneumocystis prophylaxis are given in Table 33-6.
2. Prophylaxis Against M Avium Complex Infection
Prophylaxis against M avium complex is no longer recommended in most individuals who are initiating ART, including in those with CD4+ counts less than 50 cells/mcL. The incidence of M avium complex infection is very low among those on ART. In rare cases where individuals delay ART (eg, per patient preference) whose CD4 counts fall to below 50 cells/mcL, prophylaxis against M avium complex infection should be offered. Clarithromycin (500 mg orally twice daily) and azithromycin (1200 mg orally weekly) are the recommended regimens. The azithromycin regimen is generally preferred based on high adherence and low cost. Prophylaxis against M avium complex infection may be discontinued in patients who initiate ART.
3. Prophylaxis Against Toxoplasmosis
Toxoplasmosis prophylaxis is desirable in patients with a positive IgG toxoplasma serology and CD4 counts below 100 cells/mcL. Trimethoprim-sulfamethoxazole (one double-strength tablet daily) offers good protection against toxoplasmosis, as does a combination of pyrimethamine, 25 mg orally once a week, plus dapsone, 50 mg orally daily, plus leucovorin, 25 mg orally once a week. A glucose-6-phosphate dehydrogenase (G6PD) level should be checked prior to dapsone therapy, and a methemoglobin level should be checked at 1 month. Prophylaxis can be discontinued when the CD4 cells have increased to greater than 200 cells/mcL for more than 3 months.
B. Treatment of Complications of HIV Infection
Treatment of common AIDS-related complications is detailed above and in Table 33-3. In the era prior to the use of ART, patients required lifelong treatment for many infections, including CMV retinitis, toxoplasmosis, and cryptococcal meningitis. However, among patients who have a good response to ART, maintenance therapy for opportunistic infections can be terminated. For example, in consultation with an ophthalmologist, maintenance treatment for CMV infection can be discontinued when persons receiving ART have had a sustained increase in CD4 count to greater than 100 cells/mcL for at least 3-6 months. Similar results have been observed in patients with M avium complex bacteremia, who have completed a year or more of therapy for M avium complex and have an increase in their CD4 count to 100 cells/mcL for greater than 6 months while receiving ART. Cessation of secondary prophylaxis for Pneumocystis pneumonia is described above.
Treating patients with repeated episodes of the same opportunistic infection can pose difficult therapeutic challenges. For example, patients with second or third episodes of Pneumocystis pneumonia may have developed allergic reactions to standard treatments with a prior episode. Fortunately, there are several alternatives available for the treatment of Pneumocystis infection. Trimethoprim with dapsone and primaquine with clindamycin are two combinations that often are tolerated in patients with a prior allergic reaction to trimethoprim-sulfamethoxazole and intravenous pentamidine.
C. Antiretroviral Therapy
The availability of agents that in combination suppress HIV replication (Table 33-7. Antiretroviral Therapy Agents by Class (Listed in Alphabetical Order Within Classes)) has had a profound impact on the natural history of HIV infection. Indeed, with the advent of effective ART, the life expectancy of people with HIV approaches that of people without infection when treatment is initiated early in the course of the disease and maintained.
Table 33-7. Antiretroviral therapy agents by class (listed in alphabetical order within classes).| Medication | Dose | Common Side Effects | Special Monitoring1 |
|---|---|---|---|
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | |||
| Abacavir (Ziagen) | 600 mg orally once daily | Rash, fever-if occur, rechallenge may be fatal | No special monitoring |
| Emtricitabine (Emtriva) | 200 mg orally once daily | Skin discoloration palms/soles (mild) | No special monitoring |
| Lamivudine (Epivir) | 150 mg orally twice daily or 300 mg daily | Rash, peripheral neuropathy | No special monitoring |
| Zidovudine (AZT) (Retrovir) | 600 mg orally daily in two divided doses | Anemia, neutropenia, nausea, malaise, headache, insomnia, myopathy | CBC with differential 4-8 weeks after starting AZT |
| Nucleotide Reverse Transcriptase Inhibitors (NRTIs) | |||
| Tenofovir alafenamide (TAF)/emtricitabine (Descovy) | 25 mg of TAF with 200 mg of emtricitabine once daily | Weight gain; dyslipidemia; lower but still present risk of nephrotoxicity and bone resorption | Creatinine at baseline, at 2-8 weeks, then every 3-6 months; UA and urine glucose and protein at baseline and repeated as clinically indicated; HBsAg, liver enzymes at baseline, at 2-8 weeks, then every 3-6 months, continue for months after discontinuation; consider bone densitometry |
| Tenofovir (TDF) (Viread) | 300 mg orally once daily | Kidney dysfunction, bone resorption, GI distress | Creatinine at baseline, at 2-8 weeks, then every 3-6 months; UA and urine glucose and protein at baseline and repeated as clinically indicated; consider bone densitometry |
| Nonnucleoside Reverse Transcriptase Inhibitors (NNRTIs) | |||
| Doravirine (Pifeltro) | 100 mg daily | Headache, fatigue, abdominal pain | No special monitoring |
| Efavirenz (Sustiva) | 600 mg orally daily | Neurologic disturbances, rash, hepatitis | No special monitoring |
| Etravirine (Intelence) | 200 mg orally twice daily | Rash, peripheral neuropathy | No special monitoring |
| Nevirapine (Viramune) | 200 mg orally daily for 2 weeks, then 200 mg orally twice daily | Rash | No special monitoring |
| Rilpivirine (Edurant) | 25 mg daily | Depression, rash | No special monitoring |
| Protease Inhibitors (PIs) | |||
| Atazanavir (Reyataz) | 400 mg orally once daily or 300 mg atazanavir with 100 mg ritonavir daily | Hyperbilirubinemia | Bilirubin level every 3-4 months |
| Atazanavir/cobicistat (Evotaz) | 300 mg of atazanavir with 150 mg of cobicistat orally once daily | Hyperbilirubinemia | Bilirubin level every 3-4 months |
| Darunavir/cobicistat (Prezcobix) | 800 mg of darunavir and 150 mg of cobicistat orally once daily | Rash | No special monitoring |
| Darunavir/ritonavir (Prezista/Norvir) | PI-experienced patients: 600 mg of darunavir and 100 mg of ritonavir orally twice daily For PI-naïve patients: 800 mg of darunavir and 100 mg of ritonavir orally daily | Rash | No special monitoring |
| Lopinavir/ritonavir (Kaletra) | 400 mg/100 mg orally twice daily | Diarrhea | No special monitoring |
| Ritonavir (Norvir) | 600 mg orally twice daily or in lower doses (eg, 100 mg orally once or twice daily) for boosting other PIs | GI distress, peripheral paresthesias | No special monitoring |
| Entry Inhibitors | |||
| Enfuvirtide (Fuzeon) | 90 mg subcutaneously twice daily | Injection site pain and allergic reaction | No special monitoring |
| Integrase Inhibitors | |||
| Bictegravir | 50 mg orally daily. No longer marketed as a single agent; used in antiretroviral combination (Table 33-8. Fixed-Dose Antiretroviral Combinations (Listed in Alphabetical Order by Brand Name)) | Diarrhea, nausea, headache | No special monitoring |
| Cabotegravir | Oral regimen of 30 mg daily with rilpivirine 25 mg daily for 1 month (optional); then intramuscular loading dose of 600 mg with rilpivirine 900 mg intramuscularly in separate buttock injections; followed by (1) this dosage every 8 weeks or (2) monthly intramuscular injections of 400 mg with 600 mg rilpivirine thereafter | Injection site reactions with intramuscular dose | No special monitoring |
| Dolutegravir (Tivicay) | Treatment-naïve or integrase-naïve patients: 50 mg daily When administered with efavirenz or rifampin: 50 mg twice daily When administered to integrase-experienced patients with suspected integrase resistance: 50 mg twice daily | Hypersensitivity, insomnia, fatigue, headache, rash | No special monitoring |
| Elvitegravir | No longer marketed as a single agent; used in antiretroviral combinations (Table 33-8. Fixed-Dose Antiretroviral Combinations (Listed in Alphabetical Order by Brand Name)) | Diarrhea, headache | No special monitoring |
| Raltegravir (Isentress) | 400 mg orally twice daily | Diarrhea, nausea, headache | No special monitoring |
| Entry and Fusion Inhibitors | |||
| Enfuvirtide (Fuzeon) | 90 mg subcutaneously twice daily | Injection site pain and allergic reaction | No special monitoring |
| Ibalizumab (Trogarzo) | Loading dose of 2000 mg intravenously over 30 minutes; maintenance dose of 800 mg intravenously every 2 weeks thereafter | Diarrhea, dizziness, nausea, rash, elevated creatinine, lymphopenia | Monthly CBC, creatinine, bilirubin, glucose, lipase |
| Maraviroc (Selzentry) | 150 mg orally twice daily or 300 mg orally twice daily | Cough, fever, rash | No special monitoring |
| Capsid Inhibitor | |||
| Lenacapavir | At first, 927 mg (2 vials) injected under the skin and 600 mg (2 tablets) once a day on day 1. Followed by 600 mg (2 tablets) once a day on day 2. For maintenance, 927 mg (2 vials) injected under the skin once a day every 6 months. | Subcutaneous nodules and injection site pain | No special monitoring |
| Attachment Inhibitor | |||
| Fostemsavir | 600 mg orally twice a day | Nausea | No special monitoring |
1 Standard monitoring is CBC and differential, basic chemistries, serum aminotransferases, and total bilirubin every 3-6 months, UA at baseline and annually during antiretroviral treatment, fasting glucose or hemoglobin A1c at baseline and annually during antiretroviral treatment, and fasting lipid profile at baseline, 4-8 weeks after starting an antiretroviral treatment regimen that affects lipids, and annually for everyone over 40 years of age.
The recognition that HIV damages the immune system from the beginning of infection, even when the damage is not easily measured by conventional tests, combined with the greater potency, the improved side-effect profile, and the decreased pill burden of modern HIV regimens, have led to the recommendation to start treatment as soon as possible for all people with HIV, including patients having acute HIV infection, regardless of CD4 count. The START trial demonstrated that immediate treatment is associated with a greater than 50% reduction in risk for serious illness or death, compared to delaying treatment until the CD4 count falls below 350 cells/mcL. The TEMPRANO trial showed that individuals immediately initiating ART versus delaying treatment for CD4 count to fall below 500 cells/mcL had lower rates of severe illness.
Rapid initiation programs have been created, where treatment can be started on the same day that patients test positive for HIV, so patients can start receiving treatment promptly and avoid being lost to follow-up. The clinician should provide sufficient resources to help patients cope with these major events in a short time, receive sufficient insurance and benefits coverage, and connect to other social services resources (ie, food assistance, housing, etc). If treatment is started before the results of resistance testing are available, a nonnucleoside reverse transcriptase inhibitor (NNRTI) should not be used given the possibility of transmitted drug resistance. Recommended regimens for initiating treatment before resistance testing results are available include (1) dolutegravir plus TAF/emtricitabine or TDF/emtricitabine (or lamivudine), (2) bictegravir/TAF/emtricitabine, or (3) boosted darunavir plus TAF/emtricitabine or TDF/emtricitabine (or lamivudine). The latter regimen is recommended (until resistance testing is known) if a patient developed an HIV seroconversion while on cabotegravir-based PrEP due to the possibility of the development of integrase inhibitor resistance. Also, patients requiring abacavir as part of their regimen should not start treatment prior to the results of HLA-B* 5701 allele testing or hepatitis B testing.
The primary goal of therapy should be complete suppression of viral replication as measured by the serum viral load. Partially suppressive combinations should be avoided. Similarly, if toxicity develops, it is preferable to change the offending medication given availability of multiple effective and well-tolerated medications.
Although the HIV treatment protocol has traditionally included three medications from at least two different classes, several two-drug regimens using medications from at least two different classes have been shown to be effective. A combination of dolutegravir plus lamivudine (Table 33-8. Fixed-Dose Antiretroviral Combinations (Listed in Alphabetical Order by Brand Name)) has been shown to be noninferior to dolutegravir plus TDF and emtricitabine as initial therapy in patients with HIV viral load of less than 500,000 copies/mL. A second exception is the coformulation of dolutegravir and rilpivirine (Table 33-8. Fixed-Dose Antiretroviral Combinations (Listed in Alphabetical Order by Brand Name)); this combination is FDA-approved as an alternative treatment for patients who have been successfully virally suppressed for at least 6 months, have no history of treatment failure, and are not resistant to either of the two component agents. A third approved two-drug regimen is cabotegravir with rilpivirine provided as intramuscular injections given every 4 or 8 weeks in patients who have first achieved virologic suppression on oral ART.
Table 33-8. Fixed-dose antiretroviral combinations (listed in alphabetical order by brand name).| Brand Name | Components | Dosing and Special Considerations |
|---|---|---|
| Atripla | TDF 300 mg Emtricitabine 200 mg Efavirenz 600 mg | One pill daily constitutes a complete ART regimen. |
| Biktarvy | Emtricitabine 200 mg TAF 25 mg Bictegravir 50 mg | One pill daily constitutes a complete ART regimen. One of the recommended initial treatment regimens. |
| Complera | TDF 300 mg Emtricitabine 200 mg Rilpivirine 25 mg | One pill daily constitutes a complete ART regimen. Only for patients with HIV viral load < 100,000/mL. |
| Delstrigo | TDF 300 mg Lamivudine 300 mg Doravirine 100 mg | One pill daily constitutes a complete ART regimen. |
| Descovy | TAF 25 mg Emtricitabine 200 mg | One pill daily along with an NNRTI, PI, integrase inhibitor, or maraviroc (entry inhibitor). The difference between Descovy and Truvada is that Descovy has a different form of tenofovir (TAF) that has less effect on kidney function and bone mineral density than the form of tenofovir (TDF) in Truvada. Descovy is approved for use as a single agent for PrEP in men (not studied in women). |
| Dovato | Dolutegravir 50 mg Lamivudine 300 mg | One pill daily constitutes a complete ART regimen in adults with no prior antiviral treatment and no known substitutions associated with resistance to either component. |
| Epzicom | Abacavir 600 mg Lamivudine 300 mg | One pill daily along with an NNRTI, PI, integrase inhibitor, or maraviroc (entry inhibitor). |
| Genvoya | TAF 10 mg Emtricitabine 200 mg Elvitegravir 150 mg Cobicistat 150 mg | One pill daily constitutes a complete ART regimen. Although it contains four medications, one component (cobicistat) is a medication booster only. The only difference between Stribild and Genvoya is that Genvoya has a different form of tenofovir (TAF) that appears to be safer than tenofovir TDF with less effect on kidney function and bone mineral density. |
| Juluca | Dolutegravir 50 mg Rilpivirine 25 mg | One pill daily with a meal for patients who have been virologically suppressed (viral load < 50 copies/mL) on a stable ART regimen for ≥ 6 months and no history of treatment failure or resistance to dolutegravir or rilpivirine. |
| Odefsey | TAF 25 mg Emtricitabine 200 mg Rilpivirine 25 mg | One pill daily constitutes a complete ART regimen. Only for patients with no history of HIV viral load ≥ 100,000 copies/mL. Or for replacement of stable antiretroviral regimen in patients fully suppressed for >6 months, with no history of treatment failure, and with no known resistance to components of the drug combination. |
| Stribild | TDF 300 mg Emtricitabine 200 mg Elvitegravir 150 mg Cobicistat 150 mg | One pill daily constitutes a complete ART regimen. Although it contains four medications, one component (cobicistat) is a medication booster only. |
| Symtuza | TAF 10 mg Emtricitabine 200 mg Darunavir 800 mg Cobicistat 150 mg | One pill daily constitutes a complete ART regimen. Although it contains four medications, one component (cobicistat) is a medication booster only. One of the recommended initial treatment regimens. |
| Triumeq | Abacavir 600 mg Lamivudine 300 mg Dolutegravir 50 mg | One pill constitutes a complete ART regimen. One of the recommended initial treatment regimens. |
| Trizivir | Abacavir 300 mg Lamivudine 150 mg Zidovudine 300 mg | One tablet twice daily with an NNRTI, PI, integrase inhibitor, or maraviroc (entry inhibitor). Although it contains three medications it does not constitute a complete ART regimen. |
| Truvada | TDF 300 mg Emtricitabine 200 mg | One pill daily with an NNRTI, PI, integrase inhibitor, or maraviroc (entry inhibitor). Tenofovir is the most commonly used NRTI backbone. Associated with less weight gain and lipid abnormalities than TAF. Truvada is approved for use as a single agent for PrEP. |
ART, antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor (eg, delavirdine, efavirenz, etravirine, nevirapine, rilpivirine); NRTI, nucleoside/nucleotide reverse transcriptase inhibitor (eg, abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zidovudine); PI, protease inhibitor; PrEP, pre-exposure prophylaxis; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
The presence of an acute opportunistic infection in most cases does not preclude the initiation of ART. Randomized trials compared early initiation of ART (within 2 weeks of starting treatment for an opportunistic infection or tuberculosis) with ART that was deferred until after treatment of the opportunistic infection was completed (6 weeks after its start); results demonstrated that early initiation reduced death or AIDS progression by 50%. The reduced progression rates were related to more rapid improvements in CD4 counts in patients with advanced immunodeficiency. Furthermore, IRIS and other adverse events were no more frequent in the early ART arm.
Several randomized studies have also demonstrated improved clinical outcomes in HIV/tuberculosis coinfected patients who initiate ART early in the setting of active treatment for tuberculosis and whose CD4 counts are less than 50 cells/mcL. The exception to early ART in the setting of active infections may be in patients with a CNS-associated infection, such as cryptococcal or tuberculosis meningitis. Several studies from low-income countries have shown high mortality rates with early ART initiation in this setting.
An initial antiretroviral regimen should be chosen to minimize side effects. For hospitalized patients, initiating treatment in patients with opportunistic infections requires close coordination between inpatient and outpatient clinicians to ensure that treatment is continued once patients are discharged.
D. Choosing an Antiretroviral Treatment Regimen
HIV antiretroviral medications can be grouped into six major categories: nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs); NNRTIs; PIs; integrase inhibitors; entry and fusion inhibitors; and attachment inhibitors. A capsid inhibitor administered as a subcutaneous injection (lenacapavir) every 26 weeks was FDA-approved in December 2022 for multidrug-resistant HIV in combination with oral antiretroviral agents with residual activity.
1. Nucleoside and Nucleotide Reverse Transcriptase Inhibitors
There are currently six NRTI agents available (counting TDF and TAF as separate agents) for use. The choice of which agent to use depends primarily on the patient's prior treatment experience, results of resistance testing, medication side effects, other underlying conditions, and convenience of formulation. However, most clinicians use fixed-dose combinations (see Table 33-8. Fixed-Dose Antiretroviral Combinations (Listed in Alphabetical Order by Brand Name)) of including either emtricitabine/TDF, emtricitabine/TAF, or abacavir/lamivudine (ABC/lamivudine), all of which can be given once a day. Abacavir should be given only to HLA-B* 5701-negative persons due to a risk of hypersensitivity in those who are HLA-B* 5701-positive. In patients with viral loads greater than 100,000 copies/mL, ABC/lamivudine was less effective than emtricitabine/TDF when combined with efavirenz or ritonavir-boosted atazanavir. However, ABC/lamivudine appears to be equally efficacious as emtricitabine/TDF in patients with viral loads greater than 100,000 copies/mL when combined with dolutegravir. In some studies, abacavir increased risks of MI, and therefore should be avoided in patients at high risk for CVD. Zidovudine is now rarely used due to toxicity (eg anemia or neutropenia). Of the available agents, zidovudine is the most likely to cause anemia or neutropenia. Emtricitabine, TDF, TAF, and lamivudine have activity against hepatitis B. TDF, TAF, emtricitabine, abacavir, and lamivudine can be administered once daily. Information specific to each medication is given below and in Table 33-7. Antiretroviral Therapy Agents by Class (Listed in Alphabetical Order Within Classes).
TAF attains higher levels in cells with a much lower plasma level. For this reason, it appears to cause less harm to kidneys and less bone resorption. TAF should not be used with rifamycins. TDF appears to be associated with lower lipid levels, and TAF seems to be associated with greater weight gain when combined with integrase inhibitors.
2. Nonnucleoside Reverse Transcriptase Inhibitors
NNRTIs inhibit reverse transcriptase at a site different from that of the nucleoside and nucleotide agents described above. The major advantage of NNRTIs is that four of them (efavirenz, rilpivirine, doravirine, and nevirapine) have potencies comparable to that of PIs (next section), at least for patients with viral loads under 100,000 copies/mL-with lower pill burden and fewer side effects. However, they have lower barrier to resistance when compared to PIs and integrase inhibitors. Unlike PIs, they do not cause lipodystrophy and do not seem to cause weight gain; patients with cholesterol and triglyceride elevations who are switched from a PI to an NNRTI may have improvement in their lipids. The resistance patterns of NNRTIs are distinct from those of the PIs. Because these agents may cause alterations in the clearance of PIs, dose modifications may be necessary when these two classes of medications are administered concomitantly. There is a high degree of cross-resistance among the "first-generation" NNRTIs, such that resistance to one medication in this class uniformly predicts resistance to other medications. However, the "second-generation" NNRTIs etravirine, rilpivirine, and doravirine can have consistent antiviral activity in patients with prior exposure and resistance to nevirapine or efavirenz, although genotypic analysis is needed first in these contexts. In particular, the K103N pathologic variant does not have an impact on etravirine, doravirine, or rilpivirine. There is no therapeutic reason for using more than one NNRTI at the same time.
3. Protease Inhibitors
Four PIs-ritonavir, lopinavir (in combination with ritonavir), atazanavir, and darunavir-are still available (with others used rarely). Darunavir boosted with ritonavir or cobicistat is the most commonly used PI in the United States. PIs are potent suppressors of HIV replication and are administered as part of a combination regimen.
All of the PIs-to differing degrees-are metabolized by the cytochrome P450 system, and each can inhibit and induce various P450 isoenzymes. Therefore, medication interactions are common and difficult to predict. Clinicians should consult the product inserts before prescribing PIs with other medications. Medications that are known to induce the P450 system, such as rifampin, should be avoided.
The fact that the PIs are dependent on metabolism through the cytochrome P450 system has led to the use of ritonavir to boost the medication levels of other PIs, allowing use of lower doses and simpler dosing schedules of these PIs. A second boosting agent, cobicistat, is coformulated with the PI atazanavir (Evotaz) and darunavir (Prezcobix and Symtuza). Similar to ritonavir, cobicistat also inhibits liver enzymes that metabolize other HIV medications.
All PIs have been linked to varying degrees of metabolic side effects, including elevated cholesterol levels, elevated triglyceride levels, insulin resistance, diabetes mellitus, and changes in body fat composition (eg, abdominal obesity). The lipid abnormalities and body habitus changes are referred to as lipodystrophy. Although lipodystrophy is commonly associated with PIs, it has been seen also in people with HIV who have never been treated with these agents. In particular, the lipoatrophy effects seen in patients receiving ART appear to be more related to the nucleoside toxicity and in particular to the thymidine analogs (ie, zidovudine).
Of the different manifestations of lipodystrophy, the dyslipidemias that occur are of particular concern because of the likelihood that increased levels of cholesterol and triglycerides will result in increased prevalence of heart disease. All patients taking PIs should have fasting serum cholesterol, LDL cholesterol, and triglyceride levels assessed. Clinicians should assess for CHD (see Part 30) and consider initiating dietary changes or medication therapy (or both). PIs inhibit statin metabolism. Lovastatin and simvastatin should be avoided. In general, the least interaction is with pravastatin (20 mg daily orally). Atorvastatin (10 mg daily orally) or rosuvastatin (5 mg/day orally initially; maximum 10 mg/day) may also be used cautiously. Patients with persistently elevated fasting serum triglyceride levels of 500 mg/dL or more who do not respond to dietary intervention should be treated with one of the statin medications, followed by gemfibrozil (600 mg twice daily prior to the morning and evening meals). PIs are associated with abnormalities in cardiac conduction, especially prolongation of the PR interval.
4. Integrase Inhibitors
Integrase inhibitors slow HIV replication by blocking the HIV integrase enzyme needed for the virus to multiply. They are now the preferred regimens for initiating therapy because of the combination of efficacy, ease of administration, and low incidence of side effects. Five integrase inhibitors are currently available: raltegravir; elvitegravir; dolutegravir; bictegravir; and cabotegravir, which is given via injections along with injections of rilpivirine every 4 or 8 weeks. Clinical trials of available integrase inhibitors reveal a consistent pattern of more rapid decline in viral load compared with more standard PI/r or NNRTI-based regimens. Integrase inhibitors are effective (when combined with other active medications) in the treatment of people with HIV with documented resistance to each of the three main classes of antiretroviral medications (nucleoside analogs, PIs, NNRTIs). Avoid administering oral integrase inhibitors with antacids or other medications with divalent cations (Ca2+ , Mg2+ , Al2+ , Fe2+ ) because chelation of the integrase inhibitor by the cation reduces absorption. When these medications must be taken with integrase inhibitors, they should either be taken together with food or the integrase inhibitor taken 2 hours before divalent cations. Integrase inhibitors have been associated with weight gain, with or without tenofovir alafenamide.
5. Entry and Fusion Inhibitors
6. Attachment Inhibitor
7. Capsid Inhibitor
Segal-MaurerSet al; CAPELLA Study Investigators. Capsid inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N Engl J Med. 2022;386:1793. [PMID: 35544387] |
8. Constructing an Initial Regimen
Guidelines for starting ART are shown in Table 33-9. Recommended and Alternative Initial Antiretroviral Therapy Regimens (Listed in Alphabetical Order Within Categories). The regimens with the strongest evidence all contain integrase inhibitors. This reflects their high efficacy, high barrier to resistance, tolerability, low pill burden, and safety profile. The two best-tolerated, high barrier to resistance integrase inhibitors are bictegravir and dolutegravir and so they form the backbone of the recommended regimens. Integrase inhibitors are typically given with a backbone of two NRTIs (although see discussion of two-drug therapy below). With respect to NRTIs, the most commonly used combination is TDF or TAF with emtricitabine or lamivudine. From an efficacy standpoint, there is no difference between TDF or TAF; for patients on a boosted regimen (ie, one including cobicistat or ritonavir), those with renal dysfunction or osteoporosis or osteopenia (or risk for these conditions) should receive TAF. However, TAF is associated with increased weight gain and dyslipidemia, so TDF may be considered in individuals with obesity or dyslipidemia. Also, choosing between TDF and TAF may depend on convenience: which one is coformulated with other desired partner drugs. Emtricitabine and lamivudine are essentially the same from the point of view of efficacy and side effects. The next most commonly used two-drug NRTI backbone is abacavir and lamivudine (coformulated as part of Epzicom or with dolutegravir in Triumeq). Given the need to perform B* 5701 allele and hepatitis B testing, abacavir-containing regimens are not appropriate for rapid ART initiation.
Table 33-9. Recommended and alternative initial antiretroviral therapy regimens (listed in alphabetical order within categories).| Regimen | Advantages | Disadvantages |
|---|---|---|
| Recommended Initial Regimens | ||
| Bictegravir + TAF + emtricitabine (Biktarvy) | Single pill once-a-day regimen Low risk of resistance Noninferior to dolutegravir | Less experience in heavily treated patients than with dolutegravir |
Dolutegravir (50 mg daily)1 + Either: emtricitabine/TDF or emtricitabine/TAF or lamivudine/TDF or lamivudine/TAF | Has activity in some patients with integrase resistance Once-a-day regimen Dolutegravir plus either abacavir/lamivudine or emtricitabine/TDF is superior to darunavir/ritonavir plus either of the NRTI backbones | No single tablet available When used in patients with integrase resistance or combined with certain other medications, requires twice-a-day dosing |
| Dolutegravir + abacavir + lamivudine (Triumeq) | Single pill once-a-day regimen Low risk of resistance Superior to Atripla Dolutegravir plus either abacavir/lamivudine or emtricitabine/TDF is superior to darunavir/ritonavir plus either of the NRTI backbones | Abacavir should be used only in HLA-B*5701-negative persons Should not be used in patients with hepatitis B coinfection When used in patients with integrase resistance or combined with certain other medications, requires twice-a-day dosing Fixed-dose combination should not be used in patients with creatinine clearance < 50 mL/min |
Dolutegravir + lamivudine (Dovato) | Only recommended initial two-drug regimen Single pill once-a-day regimen | Not for use in patients with HIV RNA >500,000 copies/mL, or patients initiating therapy during an opportunistic infection, or patients with hepatitis B coinfection, or patients in whom antiretroviral therapy is being started prior to results of HIV genotypic resistance testing or hepatitis B testing. |
| Other Integrase Inhibitor Regimens | ||
Raltegravir (400 mg twice daily or 1200 mg once daily) + Either: emtricitabine/TDF or emtricitabine/TAF or lamivudine/TDF or lamivudine/TAF | Fewest drug interactions of integrase inhibitors | Lower barrier to resistance than bictegravir and dolutegravir Requires twice-a-day dosing or taking two pills once a day No single tablet available |
| Alternative Initial Regimens That Are Non-Integrase Inhibitor-Based | ||
Darunavir (800 mg daily) with cobicistat + Either: emtricitabine/TDF or emtricitabine/TAF or lamivudine/TDF or lamivudine/TAF (boosted PI regimen) | Single tablet once-a-day regimen (with emtricitabine and TAF, Symtuza) | Cobicistat boosting causes similar drug-drug interactions as ritonavir; increases in serum creatinine (nonpathologic) |
Darunavir (800 mg daily) with ritonavir (100 mg daily) boosting + Either: emtricitabine/TDF or emtricitabine/TAF or lamivudine/TDF or lamivudine/TAF (boosted PI regimen) | Potent boosted PI Can be given once daily Limited risk of resistance with poor adherence | Not available as a single tablet May cause rash in patients with sulfa allergy Ritonavir boosting required Has metabolic side effects |
Doravirine with Either: emtricitabine/TDF or emtricitabine/TAF or lamivudine/TDF or lamivudine/TAF | Avoids the use of both integrase inhibitors and PIs Available as a single pill with lamivudine and TDF (Delstrigo) Does inhibit or induce the cytochrome P450 3A4 enzyme | In cases of viral virologic failure, NNRTI cross-resistance may develop. |
Rilpivirine/emtricitabine/TDF (Complera) or with emtricitabine/TAF (Odefsey) Non-integrase, non-PI regimens | Single tablet once-a-day regimens Noninferior to Atripla in patients with baseline viral load < 100,000/mL Limited metabolic side effects | Requires taking with a meal Cannot be used with PPIs Use only in patients with viral loads < 100,000 copies/mL and CD4 counts >200 cells/mcL Do not use in patients with viral loads >100,000 copies/mL or CD4 counts < 200 cells/mcL |
1 Usual medication doses are supplied when not part of a fixed-dose preparation.
NNRTI, nonnucleoside reverse transcriptase inhibitor (eg, delavirdine, efavirenz, etravirine, nevirapine, rilpivirine); NRTI, nucleoside/nucleotide reverse transcriptase inhibitor (eg, abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zidovudine); PI, protease inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
The only two-drug regimen approved for initial ART is dolutegravir plus lamivudine, which is available as a single pill for once-a-day treatment (Dovato, Table 33-8. Fixed-Dose Antiretroviral Combinations (Listed in Alphabetical Order by Brand Name)). (All other recommended first-line initial regimens contain three medications, sometimes with a fourth agent as a booster.) This two-drug regimen is not recommended for patients with high HIV viral load (greater than 500,000 copies/mL), or for patients with HBV coinfection (because of development of resistance by the hepatitis B virus to lamivudine when used as monotherapy with possibility of severe flares of hepatitis), or for patients for whom the results from HIV resistance or HBV testing are not yet available. There is also concern about its use in patients with CD4 cell counts less than 200/mL. Dolutegravir/rilpivirine (Juluca) and injectable cabotegravir/rilpivirine (Cabenuva) are approved only in the setting of prior virologic suppression without prior treatment failure or drug resistance, although there are promising observational data regarding its use among patients who are not virologically suppressed but do not have resistance to its components and cannot take oral ART.
Studies have shown dolutegravir/abacavir/lamivudine to be superior to efavirenz/TDF/emtricitabine and have shown dolutegravir to be superior to ritonavir-boosted darunavir (both combined with either abacavir/lamivudine or TDF/emtricitabine). A network meta-analysis adjusting for NRTI backbone found that dolutegravir had superior efficacy in suppressing viral load compared with regimens containing ritonavir-boosted atazanavir, ritonavir-boosted darunavir, efavirenz, or ritonavir-boosted lopinavir. Discontinuation due to adverse events was also statistically lower with the dolutegravir regimens.
The emergence of generic antiretroviral medications is also likely to affect prescribing choices when equally effective regimens are available at different costs. There are generic versions available for abacavir, atazanavir, efavirenz, lamivudine, and TDF. But how this will affect the costs patients pay can be very difficult to determine because of complicated copay rules.
For patients who cannot take an integrase inhibitor, alternative regimens are recommended (Table 33-9. Recommended and Alternative Initial Antiretroviral Therapy Regimens (Listed in Alphabetical Order Within Categories)).
Resistance testing should be performed prior to starting ART. Of people with newly diagnosed infections in some urban areas of the United States, 8-10% have transmitted drug resistance (most commonly to NNRTIs followed by NRTIs).
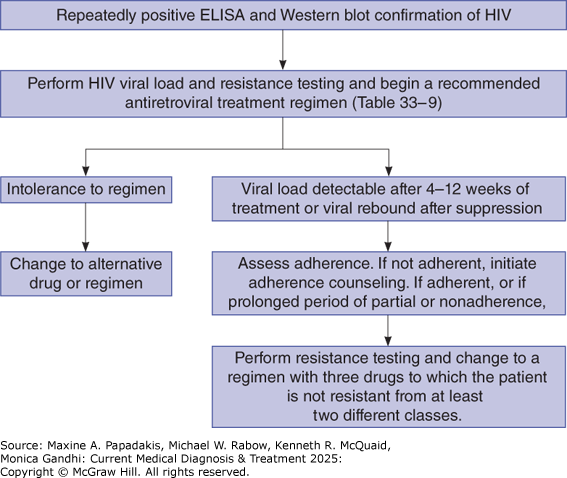
The most important determinant of treatment efficacy is adherence to the regimen. Therefore, it is vitally important that the regimen chosen be one to which the patient can easily adhere (Figure 33-7). In general, patients are more adherent if their medication regimens (Table 33-9. Recommended and Alternative Initial Antiretroviral Therapy Regimens (Listed in Alphabetical Order Within Categories)) offer complete therapy in one pill that needs to be taken only once a day, do not require special timing with regard to meals, can be taken at the same time as other medications, do not require refrigeration or special preparation, and do not have bothersome side effects. Given the high level of effectiveness of recommended regimens, patients for whom the viral load does not fully suppress are likely to be encountering adherence challenges. Pharmacists and other specially trained clinicians can be very effective in helping patients improve their adherence by taking the time to understand why patients miss their medications and to problem solve (eg, take medicine at same time every day, keep a supply in the car or at work in case they forget). Demonstration projects are examining long-acting cabotegravir and rilpivirine as injectables in people challenged by adherence or viremia, with high virologic suppression achieved in one demonstration project in San Francisco, with 97.5% virologic suppression projected. For certain populations (eg, unstably housed individuals), specially tailored programs may be beneficial.
E. Monitoring Antiretroviral Treatment
1. Goals of Monitoring Art
Monitoring of ART (Figure 33-7) has two goals: evaluate for toxicity and measure efficacy using objective markers to determine whether to maintain or change regimens. Laboratory evaluation for toxicity depends on the specific medications in the combination but generally should be done approximately every 3-6 months once a patient is on a stable regimen. Patients who are intolerant of their initial regimen should be changed to one of the other initial recommended or alternative regimens in Table 33-9. Recommended and Alternative Initial Antiretroviral Therapy Regimens (Listed in Alphabetical Order Within Categories). The second aspect of monitoring is to measure HIV viral load, the objective marker of efficacy. The HIV viral load should be repeated 2-4 weeks after the initiation or change of antiretroviral regimen and every 3-6 months thereafter in clinically stable patients. With integrase regimens, a two-log10 reduction is expected within 2 weeks of starting therapy, and approximately 80% of patients will have undetectable HIV viral load at 1 month. All patients should have undetectable viral loads by 3 months; if not, the usual problem is nonadherence (see below). CD4+ counts are most useful in determining the immunologic response to ART, although the response can vary significantly, and determining whether opportunistic infection prophylaxis can be discontinued. CD4+ counts should be monitored approximately every 3-6 months in individuals newly initiating ART. For those who have consistently suppressed HIV viral loads over the first 1-2 years of therapy with a CD4+ count greater than 300 cells/mcL, monitoring can occur yearly, and is optional in those with CD4+ counts greater than 500 cells/mcL.
2. The Challenge of Medication Adherence
In a patient who is adherent to an integrase inhibitor regimen, viral load should drop by 100-fold within 2 weeks. For patients in whom viral loads do not decrease adequately, or who have viral rebound after suppression, the major question facing the clinician is whether the patient is nonadherent or has resistance to the regimen, or both. Patients who are having trouble adhering to their treatment should receive adherence counseling. In patients who are adherent or who have missed enough doses to make resistance possible, resistance testing should be performed. Based on the results of resistance testing, if there is no treatment-emergent resistance, and the patient is tolerating the regimen well, the patient should continue the regimen with assessment of potential barriers to adherence (ie, mental health, substance use, medication coverage, or housing challenges). If resistance has emerged, the patient should be switched to a high-barrier-to-resistance regimen with at least two, but ideally three, active agents (ie, three-drug dolutegravir, bictegravir, or boosted darunavir-based regimen).
Once ART has been initiated, it is not advisable to stop the therapy. So-called drug holidays or structured treatment interruptions are not recommended because they have been shown to increase risk of AIDS-related complications, increase CD4 declines, and increase morbidity from non-AIDS-related complications (eg, MIs and liver failure).
3. The Challenge of Medication Resistance
Resistance to HIV-1 medication has been documented for all currently available antiretrovirals. Although HIV medication resistance was common in the past, high-level resistance has been declining in the past few years, likely related to better tolerated, easier to use, and more efficacious antiretroviral agents. Resistance also occurs in patients who are ART-naïve but who are infected with a medication-resistant strain-called "primary" or "transmitted" medication resistance. Cohort studies of ART-naïve patients entering care in North America and Western Europe show that roughly 8-10% of people with a recent infection have a medication-resistant strain of HIV-1.
In addition to being part of a standard baseline evaluation, resistance testing is recommended for patients who are receiving ART and have suboptimal viral suppression (typically a viral load greater than 500 copies/mL is needed for resistance testing be performed). Both genotypic and phenotypic resistance tests are commercially available, and in randomized controlled studies, genotypic resistance testing has been shown to result in improved short-term virologic outcomes compared to making treatment choices without resistance testing. Furthermore, multiple retrospective studies have conclusively demonstrated that resistance tests provide prognostic information about virologic response to newly initiated therapy that cannot be gleaned from standard clinical information (ie, treatment history, examination, CD4 count, and viral load tests).
Because of the complexity of resistance tests, many clinicians require expert interpretation of results. In the case of genotypic assays, results may show that the pathologic variants that are selected for during ART are medication-specific or contribute to broad cross-resistance to multiple medications within a therapeutic class. An example of a medication-specific pathologic variant for the reverse transcriptase inhibitors would be the M184V pathologic variant that is selected for by lamivudine or emtricitabine therapy-this pathologic variant causes resistance only to those two medications. Conversely, the thymidine analog pathologic variants ("TAMs") of M41L, D67N, K70R, L210W, T215Y/F, and T219Q/K/E are selected for by either prior zidovudine or stavudine therapy, but cause resistance to all the medications in the class and often extend to the nucleotide inhibitor tenofovir when three or more of these TAMs are present. The combination of M184V and K65R is important to know as it causes resistance to all NRTIs except zidovudine and would typically necessitate change to an NRTI-sparing regimen. The most common pathologic variants associated with medication resistance and cross-resistance patterns for NRTIs, NNRTIs, PIs, and integrase inhibitors can be found at http://hivdb.stanford.edu (see specific references below). Phenotypic resistance testing can provide complementary data and is usually provided with an interpretation about susceptibility to the virus.
Resistance results may be misleading if a patient is not taking antiretroviral medications at the time of testing because the dominant virus is likely the wild-type, even if there are resistant viruses in the body that can become dominant with the selective pressure of antivirals. Thus, resistance results do not replace a careful history of what medications a patient has taken in the past and for how long. Also, the results of resistance testing should be viewed cumulatively-ie, if resistance is reported to an agent on one test, it should be presumed to be present thereafter even if subsequent tests do not give the same result.
Stanford University HIV Drug Resistance Database Home Page (http://hivdb.stanford.edu/) provides Genotypic Resistance Interpretation Algorithm, HIVdb Program, version 9.0, February 22, 2021 (http://hivdb.stanford.edu/hivdb/by-mutations/); Genotype-Phenotype Correlations (http://hivdb.stanford.edu/pages/genotype-phenotype.html); Genotype-Treatment Correlations (http://hivdb.stanford.edu/pages/genotype-rx.html); Genotype-Clinical Outcome Correlations (http://hivdb.stanford.edu/pages/genotype-clinical.html).
F. Constructing Antiretroviral Treatment Regimens for Patients with Resistance
In designing second-line regimens for patients with resistance to initial therapy, the goal is to identify three medications from at least two different classes to which the virus is not resistant. Even without resistance testing, certain forms of cross-resistance between medications within a class can be assumed. For example, the resistance patterns of raltegravir and elvitegravir are overlapping, and patients with treatment-emergent resistance to these regimens potentially harboring resistance to second-generation integrase inhibitors such as bictegravir and dolutegravir. Fostemsavir (an attachment inhibitor), ibalizumab (a monoclonal antibody), and lenacapavir (a subcutaneously administered capsid inhibitor) are specifically FDA approved for heavily treated adults with multidrug-resistant HIV who are not responding to their existing regimen. They are used in combination with other antiretroviral drugs.
In constructing regimens, toxicities should be nonoverlapping and agents that are either virologically antagonistic or incompatible in terms of drug-drug interactions should be avoided. For example, dolutegravir and etravirine should not be coadministered without inclusion of a ritonavir-boosted PI, as etravirine will reduce the plasma concentrations of dolutegravir. The combination of TDF and boosted PIs should ideally be avoided given the potential for increased tenofovir toxicity with this regimen. Coformulated TAF and boosted PI regimens (ie, emtricitabine/TAF/darunavir or Symtuza) are preferred in this setting given these are the only formulations where a lower dose of TAF is available (10 mg versus typical 25 mg dose). Lamivudine and emtricitabine are very similar medications and so are not used together.
Given the availability of new class medications and new generation medications, a combination of ART can successfully treat virtually all patients-no matter how much resistance is present.