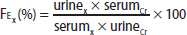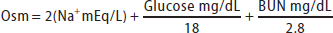The pathophysiology of all electrolyte disorders is rooted in basic principles of total body water and its distribution across fluid compartments. The optimal evaluation and treatment of fluid and electrolyte disorders requires a careful interpretation of serum and urine chemistries in conjunction with a thorough history and physical examination. While classic teaching has focused on physical examination to determine a patient's volume status, such an approach can be challenging because of limitations in accurate bedside analysis of volume status.
A. Body Water and Fluid Distribution
Total body water depends on the relative proportions of muscle and fat in the body. Total body water is typically estimated as 50% of body weight in women and 60% in men, as women, on average, have a higher proportion of fat to body weight (Table 23-1. Total Body Water (as Percentage of Body Weight) in Relation to Age and Sex). Total body water also tends to decrease with age due to declining muscle mass. Approximately two-thirds of total body water is located in the intracellular compartment and one-third is located in the extracellular compartment. The volume in the extracellular compartment is further divided into two components, specifically the interstitial fluid volume (15% of body weight) and the plasma fluid volume (5% of body weight).
Table 23-1. Total body water (as percentage of body weight) in relation to age and sex.Age | Male | Female |
|---|---|---|
18-40 | 60% | 50% |
41-60 | 60-50% | 50-40% |
Over 60 | 50% | 40% |
Changes in total body water content are best evaluated by documenting changes in body weight. Extracellular volume (ECV) may be assessed by physical examination (eg, blood pressure, pulse, jugular venous distention, peripheral or central edema). Quantitative assessments of ECV and intravascular volume may be invasive (ie, CVP assessed via a catheter or pulmonary wedge pressure from right heart catheterization) or noninvasive (ie, inferior vena cava diameter and right atrial pressure assessed by surface echocardiography). Intracellular volume (ICV) is assessed using the serum sodium concentration.
B. Serum Electrolytes
Under healthy conditions, serum electrolytes are maintained within a narrow range by the kidneys (homeostasis). The serum level of an electrolyte may be normal, elevated, or decreased but may not correlate with the total body levels of that electrolyte due to shift of water or electrolytes into and out of cells.
C. Evaluation of Urine
The urine concentration of an electrolyte is helpful to determine whether the kidney is excreting or retaining the electrolyte in response to high or low serum levels. A 24-hour urine collection for daily electrolyte excretion remains the gold standard for assessment of renal electrolyte handling; however, the collection process can be cumbersome, as well as technically challenging in certain patients. A more convenient method to determine renal electrolyte handling is the use of fractional excretion (FE) of an electrolyte X (FEx), which is calculated from a spot urine sample and concomitant serum sample, using creatinine (Cr):
A formula reads, F sub E sub x percent equals urine sub x times serum sub C r over serum sub x times urine sub C r, times 100.
A low fractional excretion indicates renal reabsorption (electrolyte retention), while a high fractional excretion indicates renal wasting (electrolyte excretion). Thus, fractional excretion helps determine whether the kidney's response is appropriate for a specific electrolyte disorder.
D. Serum Osmolality
Total solute concentration is measured by osmolality in millimoles per kilogram. Osmolarity is measured in millimoles of solute per liter of solution. The terms are often used interchangeably in clinical medicine. Plasma osmolality is the total concentration of all the solutes contained in plasma, both electrolytes and non-electrolytes, and normally ranges between 285 and 295 mOsm/kg. Differences in osmole concentration across cell membranes lead to movement of water to the region of higher osmolality. Substances that easily permeate cell membranes (eg, urea, ethanol) are ineffective osmoles and do not cause fluid shifts across fluid compartments. High serum osmolality stimulates thirst and increases secretion of antidiuretic hormone (ADH).
Serum osmolality (Osm) can be estimated using the following formula:
A formula reads, O s m equals 2 times N a with positive charge milliequivalents per liter, plus Glucose milligrams per deciliter over 18, plus begin fraction B U N milligrams per deciliter over 28.
(Note: dividing urea by 2.8 converts mg/dL to mmol/L; dividing glucose by 18 converts mg/dL to mmol/L)
Sodium is the major extracellular cation; in the body doubling the serum sodium in the formula for estimated osmolality accounts for corresponding anions. A discrepancy between measured and estimated osmolality of greater than 10 mmol/kg suggests an osmolal gap, which is the presence of unmeasured osmoles such as ethanol, methanol, isopropanol, and ethylene glycol (see Table 40-5).