Key Clinical Updates in Pneumonia Two classes of pneumococcal vaccines for adults are available and approved for use in the United States: one containing capsular polysaccharide antigens to 23 common strains of Streptococcus pneumoniae (PPSV23) and several other polyconjugate vaccines, including 13-valent (PCV13), 15-valent (PCV15), and 20-valent (PCV20). Kobayashi M et al. MMWR Recomm Rep. [PMID: 37669242] Vaccination is recommended for all adults aged 65 and over, and adults aged 19-64 with certain medical comorbidities (diabetes, chronic lung disease, chronic liver disease); increased risk of meningitis (CSF leak, cochlear implant); or immunocompromising condition, including asplenia. Kobayashi M et al. MMWR Recomm Rep. [PMID: 37669242] |
Pneumonia has classically been considered in terms of the infecting organism (Table 9-9. Characteristics of Selected Pneumonias) (eFigure 9-7) (eFigure 9-8) (eFigure 9-9). This approach facilitates discussion of characteristic clinical presentations but is a limited guide to patient management since specific microbiologic information is usually not available at initial presentation. Classification schemes emphasize epidemiologic factors that predict etiology and guide initial therapy. Pneumonia may be classified as community-acquired pneumonia (CAP) or nosocomial pneumonia and, within the latter, as hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP). These categories are based on differing settings and infectious agents and require different diagnostic and therapeutic interventions. Anaerobic pneumonia and lung abscess can occur in both hospital and community settings.
Table 9-9. Characteristics of selected pneumonias.| Organism; Appearance on Smear of Sputum | Clinical Setting | Complications |
|---|---|---|
| Streptococcus pneumoniae (pneumococcus). Gram-positive diplococci. | Chronic cardiopulmonary disease; follows upper respiratory tract infection | Bacteremia, meningitis, endocarditis, pericarditis, empyema |
| Haemophilus influenzae. Pleomorphic gram-negative coccobacilli. | Chronic cardiopulmonary disease; follows upper respiratory tract infection | Empyema, endocarditis |
| Staphylococcus aureus. Plump gram-positive cocci in clumps. | Residence in long-term care facility, hospital-associated, influenza epidemics, cystic fibrosis, bronchiectasis, injection drug use | Empyema, cavitation |
| Klebsiella pneumoniae. Plump gram-negative encapsulated rods. | Alcohol abuse, diabetes mellitus; hospital-associated | Cavitation, empyema |
| Escherichia coli. Gram-negative rods. | Hospital-associated; rarely, community-acquired | Empyema |
| Pseudomonas aeruginosa. Gram-negative rods. | Hospital-associated; cystic fibrosis, bronchiectasis | Cavitation |
| Anaerobes. Mixed flora. | Aspiration, poor dental hygiene | Necrotizing pneumonia, abscess, empyema |
| Mycoplasma pneumoniae. PMNs and monocytes; no bacteria. | Young adults; summer and fall | Skin rashes, hemolytic anemia, encephalitis |
Legionella species. Few PMNs; no bacteria. | Summer and fall; exposure to contaminated construction site, water source, air conditioner; community-acquired or hospital-associated | Empyema, cavitation, endocarditis, pericarditis |
| Chlamydophila pneumoniae. Nonspecific. | Clinically similar to M pneumoniae, but prodromal symptoms last longer (up to 2 weeks); sore throat with hoarseness common; mild pneumonia in teenagers and young adults | Reinfection in older adults with underlying COPD or HF may be severe or even fatal |
| Moraxella catarrhalis. Gram-negative diplococci. | Preexisting lung disease; older patients; corticosteroid or immunosuppressive therapy | Rarely, pleural effusions and bacteremia |
| Pneumocystis jirovecii. Nonspecific. | AIDS, immunosuppressive or cytotoxic drug therapy, cancer | Pneumothorax, respiratory failure, ARDS, death |
| SARS-CoV-2. Nonspecific. | Pandemic. Milder pneumonia (teenagers, young adults); more severe pneumonia (older, immunocompromised, multiple comorbidly ill adults) | Respiratory failure, ARDS, death |
ARDS, acute respiratory distress syndrome; PMN, polymorphonuclear leukocyte; SARS-CoV-2, severe acute respiratory syndrome due to coronavirus-2 (see COVID-19 discussion, Part 34, and consult http://www.coronavirus.gov for the latest from the CDC).
This section sets forth the evaluation and management of pulmonary infiltrates in immunocompetent persons separately from the approach to immunocompromised persons—defined as those with HIV disease, absolute neutrophil counts less than 1000/mcL (1.0 × 109 /L), or current or recent exposure to myelosuppressive or immunosuppressive medications.
1. Community-Acquired Pneumonia
| ESSENTIALS OF DIAGNOSIS | ||
|
General Considerations
Community-acquired pneumonia (CAP) is a common disorder, with approximately 4-5 million cases diagnosed each year in the United States, at least 25% of which require hospitalization. It is the deadliest infectious disease in the United States and is routinely among the top 10 causes of death. Mortality in milder cases treated as outpatients is less than 1%. Among patients hospitalized for CAP, in-hospital mortality is approximately 10-12% and 1-year mortality (in those over age 65) is greater than 40%. Risk factors for the development of CAP include older age; tobacco use; excessive alcohol use; comorbid medical conditions, especially COPD or other chronic lung disease; immunosuppression; and recent viral upper respiratory tract infection.
The patient's history, physical examination, and imaging studies are essential to establishing a diagnosis of CAP. For the identification of specific pathogen, sputum examination may be helpful, but 40% of patients cannot produce an evaluable sputum sample; additionally, test characteristics of sputum Gram stain and culture vary by organism and lack sensitivity for some of the most common causes of pneumonia. Since patient outcomes improve when the initial antibiotic choice is appropriate for the infecting organism, the American Thoracic Society and the Infectious Diseases Society of America recommend empiric treatment based on epidemiologic data (Table 9-10. Recommended Empiric Antibiotics for Community-Acquired Bacterial Pneumonia). Such treatment improves initial antibiotic coverage, reduces unnecessary hospitalization, and improves 30-day survival.
Table 9-10. Recommended empiric antibiotics for community-acquired bacterial pneumonia.Outpatient management
| |||
Inpatient management of nonsevere pneumonia (typically not requiring intensive care)
| |||
Inpatient management of severe pneumonia (typically requiring intensive care). All agents administered intravenously, except as noted.
|
MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus.
Recommendations assembled from Metlay JP et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67.
Definition & Pathogenesis
CAP is diagnosed outside of the hospital setting or within the first 48 hours of hospital admission. Pulmonary defense mechanisms (cough reflex, mucociliary clearance system, immune responses) normally prevent the development of lower respiratory tract infections following aspiration of oropharyngeal secretions containing bacteria or inhalation of infected aerosols. CAP occurs when there is a defect in one or more of these normal defense mechanisms or when a large infectious inoculum or a virulent pathogen overwhelms the immune response.
Prospective studies fail to identify the cause of CAP in 30-60% of cases; two or more causes are identified in up to one-third of cases. The most common bacterial pathogen identified in most studies of CAP is S pneumoniae, accounting for approximately two-thirds of bacterial isolates. Other common bacterial pathogens include H influenzae, M pneumoniae, C pneumoniae, S aureus, Moraxella catarrhalis, Klebsiella pneumoniae, other gram-negative rods, and Legionella species. Common viral causes of CAP include coronaviruses (SARS-CoV-2, MERS), influenza virus, RSV, adenovirus, and parainfluenza virus. A detailed assessment of epidemiologic risk factors may aid in diagnosing pneumonias due to the following uncommon causes: Chlamydophila psittaci (psittacosis); Coxiella burnetii (Q fever); Francisella tularensis (tularemia); Blastomyces, Coccidioides, Histoplasma (endemic fungi); and Sin Nombre virus (hantavirus pulmonary syndrome).
Clinical Findings
A. Symptoms and Signs
Most patients with CAP experience an acute or subacute onset of fever, cough with or without sputum production, and dyspnea. Other common symptoms include chills, rigors, pleurisy, fatigue, myalgias, anorexia, headache, and abdominal pain. Persons over age 80, however, may have an atypical presentation, including falls, delirium, lethargy, and anorexia.
Common physical findings include fever or hypothermia, tachypnea, and tachycardia. Many patients appear acutely ill. Chest examination often reveals inspiratory crackles, rhonchi, and bronchial breath sounds. Dullness to percussion may be observed if lobar consolidation or a parapneumonic pleural effusion is present. The clinical evaluation is less than 50% sensitive compared to chest imaging for the diagnosis of CAP (see Imaging Otolaryngology Disorders section below). In most patients, therefore, imaging is essential to the evaluation of suspected CAP.
B. Diagnostic Testing
Diagnostic testing for a specific infectious cause of CAP is not generally indicated in outpatients because empiric antibiotic therapy is almost always effective in this population. In ambulatory outpatients whose presentation (travel history, exposure) suggests an etiology not covered by standard therapy (eg, Coccidioides) or public health concerns (eg, SARS-CoV-2, Mycobacterium tuberculosis, influenza), diagnostic testing is appropriate. Diagnostic testing is recommended in hospitalized CAP patients for multiple reasons: the likelihood of an infectious cause unresponsive to standard therapy is higher in more severe illness, the inpatient setting allows narrowing of antibiotic coverage as specific diagnostic information is available, and the yield of testing is higher in more acutely ill patients.
Diagnostic tests are used to adjust empirically chosen therapy and to facilitate epidemiologic analysis. Three widely available diagnostic tests may guide therapy: the sputum Gram stain and culture, urinary antigen tests for S pneumoniae and Legionella species, and tests for viruses such as influenza and SARS-CoV-2 (see COVID-19 discussion, Part 34). The usefulness of a sputum Gram stain lies in broadening initial coverage in patients hospitalized for CAP, most commonly to cover S aureus (including community-acquired methicillin-resistant S aureus [CA-MRSA] strains) or gram-negative rods (including P aeruginosa and Enterobacteriaceae). Urinary antigen assays for Legionella pneumophila and S pneumoniae are at least as sensitive and specific as sputum Gram stain and culture. Results of antigen testing are not affected by initiation of antibiotic therapy, and positive tests may allow narrowing of initial antibiotic coverage. Urinary antigen assay for S pneumoniae should be ordered for patients with leukopenia or asplenia or those with severe disease. Urinary antigen assay for L pneumophila should be ordered for patients in an area with an outbreak, with recent travel, with severe disease, or in whom a high clinical index of suspicion exists. Rapid influenza and SARS-CoV-2 testing has intermediate sensitivity but high specificity, with sensitivity depending on the method of detection (nucleic acid or PCR-based tests have higher sensitivity than antigen-based detection). Positive tests for viruses may direct isolation of hospitalized patients but do not necessarily reduce the need for antibacterial therapy, since coinfection with bacterial pathogens may occur.
Rapid turnaround multiplex-PCR amplification from lower respiratory tract samples is increasingly available. Different commercial products can identify multiple strains of bacteria and viruses, in addition to genes that encode for antibiotic resistance. Early experience with multiplex-PCR shows improved overall diagnostic yield, particularly for viral infections, and a higher incidence of bacterial/viral coinfection than previously recognized. Limitations of multiplex-PCR include cost and availability, in addition to the challenge of interpreting potentially false-positive results from a highly sensitive test, since either viral or bacterial pathogens may colonize the airways. Large scale studies evaluating the impact of rapid molecular diagnostic approaches are in progress and will help inform future utility.
Additional microbiologic testing including pre-antibiotic sputum and blood cultures (at least two sets at separate sites) has been standard practice for patients with CAP who require hospitalization. The yield of blood and sputum cultures is low, as false-positive results are common, and the impact of culture results on patient outcomes is small. As a result, targeted testing is recommended for patients with severe disease and for those treated empirically for MRSA or P aeruginosa infection. The role of culture is to allow narrowing of initial empiric antibiotic coverage, adjustment of coverage based on specific antibiotic resistance patterns, to identify unsuspected pathogens not covered by initial therapy, and to provide information for epidemiologic analysis. Patients at risk for or treated empirically for MRSA should have an MRSA nasal screening test performed. A negative nasal screening test may guide de-escalation of empiric MRSA coverage in patients who cannot expectorate a sputum sample.
Apart from microbiologic testing, hospitalized patients should undergo CBC with differential and a chemistry panel (including serum glucose, electrolytes, BUN, creatinine, bilirubin, and liver enzymes). Hypoxemic patients should have ABGs sampled. Test results help assess severity of illness and guide evaluation and management. HIV testing should be considered in all adult patients and performed in those with risk factors.
C. Imaging
A pulmonary opacity on CXR or CT scan is required to establish a diagnosis of CAP. Chest CT scan is more sensitive and specific than CXR and may be indicated in selected cases. Radiographic findings range from patchy airspace opacities to lobar consolidation with air bronchograms to diffuse alveolar or interstitial opacities. Additional findings can include pleural effusions and cavitation. Chest imaging cannot identify a specific microbiologic cause of CAP; no pattern of radiographic abnormalities is pathognomonic of any infectious cause. Chest ultrasound, which may be more readily available in resource-constrained settings, has demonstrated variable sensitivity and specificity for pneumonia when compared to CT imaging.
Chest imaging may help assess severity and response to therapy over time. Progression of pulmonary opacities during antibiotic therapy or lack of radiographic improvement over time are poor prognostic signs and raise concerns about secondary or alternative pulmonary processes. Clearing of pulmonary opacities in patients with CAP can take 6 weeks or longer. Clearance is usually quickest in younger patients, nonsmokers, and those with single-lobe involvement.
D. Special Examinations
Patients with CAP who have significant pleural fluid collections may require diagnostic thoracentesis (with pleural fluid sent for glucose, LD, and total protein levels; leukocyte count with differential; pH determination; and Gram stain and culture). Positive pleural cultures or features of complex parapneumonic effusion indicate the need for tube thoracostomy drainage.
Patients with cavitary opacities should have sputum fungal and mycobacterial cultures.
Sputum induction or fiberoptic bronchoscopy to obtain samples of lower respiratory secretions are indicated in patients with a worsening clinical course who cannot provide expectorated sputum samples or who may have pneumonia caused by M tuberculosis infection or certain opportunistic infections, including Pneumocystis jirovecii.
Procalcitonin is a biomarker that is typically associated with bacterial rather than viral infection; however, studies have not found a threshold at which bacterial pneumonia can be reliably distinguished from viral pneumonia. Therefore, procalcitonin is not recommended as a “rule-out” test for bacterial pneumonia, and empiric antibacterial agents are recommended regardless of procalcitonin level at time of presentation. In patients without other complicating features (pleural effusion, bacteremia, identified pathogen requiring longer antibiotic course), the kinetics of decline in procalcitonin level may be used to discontinue antibiotic therapy after a threshold duration of antibiotics (at least 5 days) has been met; such use decreases antibiotic exposure, with possible improvement in mortality.
Differential Diagnosis
The differential diagnosis of lower respiratory tract infection is extensive and includes upper respiratory tract infections, reactive airway diseases, HF, interstitial pneumonias, lung cancer, pulmonary vasculitis, pulmonary thromboembolic disease, and atelectasis.
Treatment
Two general principles guide antibiotic therapy once the diagnosis of CAP is established: prompt initiation of a medication to which the etiologic pathogen is susceptible.
In patients who require specific diagnostic evaluation, sputum and blood culture specimens should be obtained prior to initiation of antibiotics. Since early administration of antibiotics to acutely ill patients is associated with improved outcomes, obtaining other diagnostic specimens or test results should not delay the initial dose of antibiotics.
Optimal antibiotic therapy would be pathogen directed, but a definitive microbiologic diagnosis is not typically available on presentation. A syndromic approach to therapy, based on clinical presentation and chest imaging, does not reliably predict the microbiology of CAP. Therefore, initial antibiotic choices are empiric, based on acuity (treatment as an outpatient, inpatient, or in the ICU), patient risk factors for specific pathogens, and local antibiotic resistance patterns (Table 9-10. Recommended Empiric Antibiotics for Community-Acquired Bacterial Pneumonia).
Since S pneumoniae remains a common cause of CAP in all patient groups, local prevalence of drug-resistant S pneumoniae significantly affects initial antibiotic choice. Prior treatment with one antibiotic in a pharmacologic class (eg, beta-lactam, macrolide, fluoroquinolone) predisposes to the emergence of drug-resistant S pneumoniae, with resistance developing against that class of antibiotics to which the pathogen was previously exposed. Current in vivo efficacy appears to justify maintaining macrolides as first-line therapy except in areas where there is a high prevalence of resistant strains. Macrolide resistance has increased (approximately one-third of S pneumoniae isolates show in vitro resistance); however, reported treatment failures remain rare compared to the number of patients treated. S pneumoniae resistance to fluoroquinolones is rare in the United States but is increasing.
CA-MRSA is genetically and phenotypically different from hospital-acquired MRSA strains and is more virulent. CA-MRSA is a rare cause of necrotizing pneumonia, empyema, respiratory failure, and shock; it appears to be associated with prior influenza infection. Linezolid may be preferred to vancomycin in treatment of CA-MRSA pulmonary infection due to bacterial toxin inhibition. Daptomycin should not be used in any MRSA pneumonia because it does not achieve adequate concentration in the lung. For expanded discussions of specific antibiotics, see Parts 32 and e1.
A. Treatment of Outpatients
See Table 9-10. Recommended Empiric Antibiotics for Community-Acquired Bacterial Pneumonia for specific medication dosages. The most common etiologies of CAP in outpatients who do not require hospitalization are S pneumoniae; M pneumoniae; C pneumoniae; and respiratory viruses, including influenza. For previously healthy patients with no recent (90 days) use of antibiotics, the recommended treatment is amoxicillin, a macrolide (clarithromycin or azithromycin), or doxycycline. In areas with a high incidence of macrolide-resistant S pneumoniae, initial therapy in patients with no comorbidities may include the combination of a beta-lactam plus a macrolide, or a respiratory fluoroquinolone.
In outpatients with chronic heart, lung, liver, or kidney disease; diabetes mellitus; alcohol use disorder; malignancy; or asplenia or who received antibiotic therapy within the past 90 days, the recommended treatment is a macrolide or doxycycline plus a beta-lactam (high-dose amoxicillin and amoxicillin-clavulanate are preferred to cefpodoxime and cefuroxime) or monotherapy with a respiratory fluoroquinolone (moxifloxacin, gemifloxacin, or levofloxacin).
The default duration of antibiotic therapy for CAP should be 5 days; factors that may affect therapy duration are clinical stability, etiology (MRSA and P aeruginosa require minimum 7 days of therapy, for example), severity of illness, complications, and comorbid medical problems.
B. Treatment of Hospitalized and ICU Patients
Almost all patients admitted to a hospital for treatment of CAP receive intravenous antibiotics. However, no studies in hospitalized patients demonstrated superior outcomes with intravenous antibiotics compared with oral antibiotics, provided patients were able to tolerate oral therapy and the medication was well absorbed. Duration of inpatient antibiotic treatment is the same as for outpatients.
The most common etiologies of CAP in patients who require admission to intensive care are S pneumoniae, Legionella species, H influenzae, Enterobacteriaceae species, S aureus, Pseudomonas species, and respiratory viruses. First-line antibacterial therapy in ICU patients with CAP is an anti-pneumococcal beta-lactam (cefotaxime, ceftriaxone, ceftaroline, or ampicillin-sulbactam) plus either azithromycin or a respiratory fluoroquinolone (moxifloxacin, gemifloxacin, or levofloxacin).
Risk factors for Pseudomonas, Enterobacteriaceae, or MRSA infection must be considered when choosing empiric antibiotic therapy for inpatients with CAP. Specific risk factors for these organisms include (1) prior isolation of the pathogen, (2) inpatient hospitalization within the last 90 days, or (3) exposure to intravenous antibiotics within the last 90 days. In patients with specific risk factors for Pseudomonas infection, combine an anti-pneumococcal, anti-pseudomonal beta-lactam (piperacillin-tazobactam, cefepime, imipenem, meropenem) with either azithromycin or a respiratory fluoroquinolone (moxifloxacin, gemifloxacin, or levofloxacin). In critically ill patients, in those at increased risk for drug resistance, or if the unit incidence of monotherapy-resistant Pseudomonas is greater than 10%, consider use of two agents with anti-pseudomonal efficacy: either ciprofloxacin or levofloxacin plus the above anti-pneumococcal, anti-pseudomonal beta-lactam or an anti-pneumococcal, anti-pseudomonal beta-lactam plus an aminoglycoside (gentamicin, tobramycin, amikacin) plus either azithromycin or a respiratory fluoroquinolone. Patients with specific risk factors for MRSA should be treated with vancomycin or linezolid. Patients with very severe disease (respiratory failure requiring mechanical ventilation or septic shock) should also be strongly considered for MRSA therapy. Provided the patient is clinically improving, negative sputum and blood cultures obtained prior to initiation of antibiotics can support de-escalation of antibiotic therapy. Additionally, all patients prescribed vancomycin or linezolid should have swabs of the nasal passages for MRSA; if the swab results are negative, MRSA coverage can be safely de-escalated, even when adequate sputum samples have not been obtained.
Patients with CAP in whom influenza is detected should be treated with the antiviral oseltamivir, whether influenza is identified as a single pathogen or as a coinfection along with a bacterial pathogen. Oseltamivir is most effective when begun within 2 days but may still be beneficial within several days after symptom onset, particularly in severe cases of CAP.
Prevention
Pneumococcal vaccines prevent or lessen the severity of pneumococcal infections in immunocompetent patients. Two classes of pneumococcal vaccines for adults are available and approved for use in the United States: one containing capsular polysaccharide antigens to 23 common strains of S pneumoniae (PPSV23) and several other polyconjugate vaccines, including 13-valent (PCV13), 15-valent (PCV15), and 20-valent (PCV20).
Vaccination is recommended for all adults aged 65 and over, and adults aged 19-64 with certain medical comorbidities (diabetes, chronic lung disease, chronic liver disease), increased risk of meningitis (CSF leak, cochlear implant), or immunocompromising condition, including asplenia. Updated recommendations from the Advisory Committee on Immunization Practices published by the CDC, vary by age, underlying condition, and receipt of prior vaccination.
Adults 65 years or older: PCV20 alone is recommended for all adults 65 years or older with or without chronic medical or immunocompromising conditions. Alternatively, patients may be given sequential administration of PCV15 followed by PPSV23 injection at least one year later. For adults 65 years or older who have an immunocompromising condition or cochlear implant, PPSV23 should be administered 8 or more weeks following PCV15 injection. Many patients have already received a pneumococcal vaccine or a series of vaccines. Adults (with or without underlying medical conditions) who previously received PPSV23 alone should receive either PCV20 or PCV15 1 year or later after the PPSV23 injection. Adults who previously received PCV13 and are without immunocompromising conditions alone should receive PCV20 or PPSV23, at least 1 year later; those with underlying immunosuppression or cochlear implant should receive the PPSV23 injection 8 or more weeks following the PCV13 injection. Adults 65 years or older who received both PCV13 AND PPSV23 prior to age 65 should receive a single dose of PVC20 5 or more years after the last vaccine.
Adults 19 years or older with immunocompromising conditions or medical comorbidities: PCV20 alone is recommended for adults with immunocompromising conditions, chronic renal failure, asplenia, or increased risk of meningitis (CSF leak or cochlear implant). Alternatively, they may receive the sequential PCV15/PPSV23, with the PPSV23 administered at a shortened interval of 8 or more weeks. Adults 19-64 with high-risk conditions who previously received PCV13 or PPSV23 should receive a single dose of PVC20 1 or more years later. Adults 19-64 years who previously received both PCV13 and PPSV23 should receive PCV20 or more years following the last vaccine. PPSV23 is an acceptable alternative if PCV20 is not available.
Subsequent pneumococcal ‘revaccination' after the first vaccine at or after age 65 remains controversial. Immunocompromised patients and those at high risk of fatal pneumococcal infection should receive updated vaccination with PCV20 5 years after the last vaccination, regardless of age.
Influenza vaccine is effective in preventing severe disease due to influenza virus with a resulting positive impact on both primary influenza pneumonia and secondary bacterial pneumonias. The seasonal influenza vaccine is recommended annually for all persons older than 6 months without contraindications, with priority given to persons at risk for complications of influenza infection (persons aged 50 years or older, immunocompromised persons, residents of long-term care facilities, patients with pulmonary or cardiovascular disorders, pregnant persons) as well as health care workers and others who may transmit influenza to high-risk patients. Quadrivalent vaccination is recommended for adults 65 and older.
SARS-CoV-2 vaccinations are recommended for everyone older than 6 months without contraindications. Vaccinations (including boosters) reduce the likelihood of infection, pneumonia, hospitalization, and mortality (see COVID-19 discussion, Part 34).
RSV vaccination was recently approved for adults over age 60, especially those with chronic underlying medical conditions or deemed to be at increased risk for severe disease, using a shared-decision making model. Pregnant persons who are 32-36 weeks gestation during RSV season are recommended to receive the vaccine, as a measure to prevent severe RSV disease in the infant.
When to Admit
Once a diagnosis of CAP is made, the first management decision is to determine the site of care: Is it safe to treat the patient at home or does he or she require hospital or intensive care admission? There are two widely used clinical prediction rules available to guide admission and triage decisions, the Pneumonia Severity Index (PSI) and the CURB-65.
A. Hospital Admission Decision
The PSI is a validated prediction model that uses 20 items from demographics, medical history, physical examination, laboratory results, and imaging to stratify patients into five risk groups. In conjunction with clinical judgment, it facilitates safe decisions to treat CAP in the outpatient setting. An online PSI risk calculator is available at http://www.thecalculator.co/health/Pneumonia-Severity-Index-(PSI)-Calculator-977.html. The CURB-65 assesses five simple, independent predictors of increased mortality (Confusion, Uremia, Respiratory rate, Blood pressure, and age greater than 65) to calculate a 30-day predicted mortality (http://www.mdcalc.com/curb-65-score-pneumonia-severity). Compared with the PSI, the simpler CURB-65 is less discriminating at low mortality but excellent at identifying patients with high mortality who may benefit from ICU-level care. A modified version (CRB-65) dispenses with BUN and eliminates the need for laboratory testing.
Hospital admission decision should also include circumstances of care independent of pneumonia severity, including comorbidities and the patient's ability to care for themselves effectively at home.
B. ICU Admission Decision
Expert opinion has defined major and minor criteria to identify patients at high risk for death. Major criteria are septic shock with need for vasopressor support and respiratory failure with need for mechanical ventilation. Minor criteria are respiratory rate of 30 breaths or more per minute, hypoxemia (defined as PaO2/FIO2 equaling 250 or less), hypothermia (core temperature less than 36.0°C), hypotension requiring aggressive fluid resuscitation, confusion/disorientation, multilobar pulmonary opacities, leukopenia due to infection with WBC less than 4000/mcL (less than 4.0 × 109 /L), thrombocytopenia with platelet count less than 100,000/mcL (less than 100 × 109 /L), uremia with BUN of 20 mg/dL or more (7.1 mmol/L or more), metabolic acidosis, or elevated serum lactate level. Either one major criterion or three or more minor criteria of illness severity generally require ICU-level care.
DequinPFet al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388:1931. [PMID: 36942789] EvansSEet al. Nucleic acid-based testing for noninfluenza viral pathogens in adults with suspected community-acquired pneumonia. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2021;203:1070. [PMID: 33929301] KobayashiMet al. Pneumococcal vaccine for adults aged >=19 years: recommendations of the Advisory Committee on Immunization Practices, United States, 2023. MMWR Recomm Rep. 2023;72:1. [PMID: 37669242] MelgarMet al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Weekly Rep. 2023;72:793. [PMID: 37471262] MetlayJPet al. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45. [PMID: 31573350] MohantySet al. A multicenter evaluation of trends in antimicrobial resistance among Streptococcus pneumoniae isolates from adults in the United States. Open Forum Infect Dis. 2022;9:ofac420. [PMID: 36168549] RamirezJAet al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. 2020;158:1896. [PMID: 32561442] |
2. Nosocomial Pneumonia (Hospital-Acquired & Ventilator-Associated)
| ESSENTIALS OF DIAGNOSIS | ||
|
General Considerations
Hospitalized patients carry different flora with different resistance patterns than healthy patients in the community, and their health status may place them at higher risk for more severe infection. The diagnostic approach and antibiotic treatment of patients with HAP is, therefore, different from patients with CAP. Similarly, management of patients in whom VAP develops following endotracheal intubation and mechanical ventilation should address issues specific to this group of patients.
Considered together, nosocomial pneumonias (HAP or VAP) represent an important cause of morbidity and mortality despite widespread use of preventive measures, advances in diagnostic testing, and potent antimicrobial agents. HAP is one of the most common causes of infection among hospital inpatients and carries the highest burden of morbidity and mortality. Patients in ICUs and those who are being mechanically ventilated are at the highest risk for HAP (and VAP) and experience higher morbidity and mortality than other inpatients. Definitive identification of the infectious cause of a lower respiratory infection is rarely available on presentation; initial antibiotic treatment is therefore empiric and informed by epidemiologic and patient data rather than pathogen directed.
Definition & Pathogenesis
HAP develops more than 48 hours after admission to the hospital, and VAP develops in a mechanically ventilated patient more than 48 hours after endotracheal intubation. Three factors distinguish nosocomial pneumonia from CAP: (1) different infectious causes; (2) different antibiotic susceptibility patterns, specifically, a higher incidence of drug resistance; and (3) poorer underlying health status of patients, putting them at risk for more severe infections. Since access to the lower respiratory tract occurs primarily through microaspiration, nosocomial pneumonia starts with a change in upper respiratory tract flora. Colonization is promoted by exogenous factors (eg, instrumentation of the upper airway with nasogastric and endotracheal tubes; contact with personnel, equipment, and contaminated aerosols; treatment with broad-spectrum antibiotics that promote the emergence of drug-resistant organisms); and patient factors (eg, malnutrition, advanced age, altered consciousness, swallowing disorders, and underlying pulmonary and systemic diseases). Impaired cellular and mechanical defense mechanisms in the lungs of hospitalized patients raise the risk of infection after aspiration has occurred.
Gastric acid may play a role in protection against nosocomial pneumonias. Observational studies have suggested that elevation of gastric pH due to antacids, H2-receptor antagonists, PPIs, or enteral feeding is associated with gastric microbial overgrowth, tracheobronchial colonization, and HAP/VAP. Meta-analysis of RCTs suggested an increased risk of HAP among enterally fed patients receiving stress ulcer prophylaxis. The IDSA and other professional organizations recommend that acid-suppressive medications (H2-receptor antagonists and PPIs) be given only to patients at high risk for stress gastritis.
The microbiology of the nosocomial pneumonias differs from CAP but is substantially the same among HAP and VAP. The most common organisms responsible for HAP and VAP include S aureus (both methicillin-sensitive S aureus and MRSA), Streptococcus species, P aeruginosa, other gram-negative rods, including extended spectrum beta-lactamase (ESBL)-producing organisms (Enterobacter species, K pneumoniae, and Escherichia coli) and non-ESBL-producing organisms. VAP patients may be infected with Acinetobacter species and S maltophilia. Anaerobic organisms (Bacteroides, anaerobic streptococci, Fusobacterium) may also cause pneumonia in hospitalized patients; when these organisms are isolated, they are commonly part of a polymicrobial flora. VAP occurring before hospital day 4 in a previously healthy person with no antibiotic exposure is more likely to involve oral flora with minimal resistance profiles than multidrug-resistant pathogens. However, multidrug-resistant pathogens may complicate early-onset VAP in patients with antibiotic exposure in preceding 90 days, recent hospitalization, or prior colonization with multidrug-resistant pathogens.
Clinical Findings
A. Symptoms and Signs
The symptoms and signs associated with nosocomial pneumonias are nonspecific. However, two or more clinical findings (fever, leukocytosis, purulent sputum, worsening respiratory status) along with new or progressive pulmonary opacities on chest imaging are characteristic features of nosocomial pneumonia. Other findings include those listed above for CAP.
The differential diagnosis of new lower respiratory tract symptoms and signs in hospitalized patients includes HF, atelectasis, aspiration, ARDS, pulmonary thromboembolism, pulmonary hemorrhage, and medication reactions.
B. Laboratory Findings
Diagnostic evaluation for suspected nosocomial pneumonia includes blood cultures from two different sites. Blood cultures can identify the pathogen in 15-20% of patients with nosocomial pneumonias; positivity is associated with increased risk of complications and other sites of infection. Blood counts and clinical chemistry tests do not establish a specific diagnosis; however, they help define the severity of illness and identify complications. Thoracentesis for pleural fluid analysis should be considered in patients with pleural effusions.
Examination of lower respiratory tract secretions is attended by the same disadvantages as in CAP. Gram stains and cultures of sputum are neither sensitive nor specific in the diagnosis of nosocomial pneumonias; sensitivity of sputum decreases following antibiotic therapy, particularly after 72 hours of antibiotics. The identification of a bacterial organism by culture of lower respiratory tract secretions does not prove that the organism is a lower respiratory tract pathogen; however, it can be used to help identify bacterial antibiotic sensitivity patterns and as a guide to adjusting empiric therapy. Nasal swab for PCR detection of MRSA is recommended to guide de-escalation of broad-spectrum antibiotic therapy in patients with HAP and VAP.
C. Imaging
Radiographic findings in HAP/VAP are nonspecific and often confounded by other processes that led to hospitalization or ICU admission. (See CAP above.) Imaging may demonstrate complicating features including effusion, cavitation, or barotrauma.
D. Special Examinations
When HAP is suspected in a patient who subsequently requires mechanical ventilation, secretions may be obtained by spontaneous expectoration, sputum induction, nasotracheal suctioning, and endotracheal aspiration (qualitative or semiquantitative samples), or more invasively via bronchoscopic sampling of the lower airways (quantitative samples). The best approach remains a matter of debate, since qualitative or semiquantitative samples are more likely to return nonpathogenic organisms and are, thus, associated with higher antibiotic exposure (without improvement in mortality), while invasive quantitative sampling increases cost and patient risk. Invasive qualitative sampling is recommended when the patient does not improve during initial therapy directed at expected or isolated pathogens, or in immunocompromised persons in whom an opportunistic pathogen is suspected.
Treatment
The initial treatment of HAP and VAP is based on risk factors for MRSA and multiple drug-resistant pathogens (Table 9-11. Risk Factors for Multidrug-Resistant (Mdr) Pathogens, Methicillin-Resistant Staphylococcus Aureus (MRSA), and Pseudomonas and other Gram-Negative Bacilli in Patients with Hospital-Acquired Pneumonia (HAP) and Ventilator-Associated Pneumonia (Vap)) as well as local antibiograms and mortality risk and is thus empiric (Table 9-12. Recommended Initial Empiric Antibiotics for Hospital-Acquired Pneumonia (HAP) and Ventilator-Associated Pneumonia (Vap)). The predictive capability of sets of risk factors for drug-resistant organisms in nosocomial pneumonia vary locally; strongest predictive factors include prior isolation of drug-resistant organisms, a high local prevalence of drug-resistant organisms, and antibiotic exposure within prior 90 days. Each hospital should generate antibiograms to guide the optimal choice of antibiotics with the goals of reducing exposure to unnecessary antibiotics and the development of antibiotic resistance. Because of the high mortality rate, therapy should be started as soon as HAP or VAP is suspected. After results of cultures are available, it may be possible to narrow initially broad therapy to more specific agents. Endotracheal aspiration cultures have significant negative predictive value but limited positive predictive value in the diagnosis of specific infectious causes of HAP/VAP. If an invasive diagnostic approach to suspected VAP using quantitative culture of bronchoalveolar lavage (BAL), protected specimen brush (PSB), or blind bronchial sampling (BBS) is used, antibiotics can be withheld when results are below a diagnostic threshold (BAL less than 104 CFU/mL, PSB or BBS less than 103 CFU/mL). Duration of antibiotic therapy is 7 days, consistent with clinical response, but should be individualized based on the pathogen, complications (empyema, necrotizing pneumonia), severity of illness, response to therapy, and comorbid conditions.
Table 9-11. Risk factors for multidrug-resistant (MDR) pathogens, methicillin-resistant Staphylococcus aureus (MRSA), and Pseudomonas and other gram-negative bacilli in patients with hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP).Risk factors for MDR pathogens Antibiotic therapy in the preceding 90 days Septic shock Acute respiratory distress syndrome preceding VAP ≥ 5 days in hospital prior to occurrence of HAP/VAP Acute renal replacement therapy prior to HAP/VAP onset Treatment in a unit where >10% of gram-negative isolates are resistant to an agent being considered for monotherapy Treatment in a unit where local antibiotic susceptibility rates are not known Risk factors for MRSA Antibiotic therapy in the preceding 90 days Renal replacement therapy in the preceding 30 days Use of gastric acid suppressive agents Positive culture or prior MRSA colonization, especially in the preceding 90 days Hospitalization in a unit where >20% of S aureus isolates are MRSA Hospitalization in a unit where prevalence of MRSA is not known Risk factors for Pseudomonas aeruginosa and other gram-negative bacilli Antibiotic therapy in the preceding 90 days Structural lung disease (COPD, especially with recurrent exacerbations; bronchiectasis; or cystic fibrosis) Recent hospitalizations, especially with manipulation of the aerodigestive tract (nasoenteric nutrition, intubation) High-quality Gram stain of respiratory secretions with numerous and predominant gram-negative bacilli Positive culture for P aeruginosa in the past year |
Data from Kalil AC et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61.
HAP not at high risk for mortality, or VAP with no risk factors for MRSA, MDR, or Pseudomonas and other gram-negative bacilli USE one of the following: Piperacillin-tazobactam, 4.5 g intravenously every 6 hours1 Cefepime, 2 g intravenously every 8 hours1 Levofloxacin, 750 mg intravenously daily Imipenem, 500 mg intravenously every 6 hours1 Meropenem, 1 g intravenously every 8 hours1 | |||
HAP or VAP with risk factors for MRSA but no risk factors for MDR, Pseudomonas, and other gram-negative bacilli USE one of the following: Piperacillin-tazobactam, 4.5 g intravenously every 6 hours1 Cefepime, 2 g intravenously every 8 hours1 Ceftazidime, 2 g intravenously every 8 hours Levofloxacin, 750 mg intravenously daily Ciprofloxacin, 400 mg intravenously every 8 hours Imipenem, 500 mg intravenously every 6 hours1 Meropenem, 1 g intravenously every 8 hours1 Aztreonam, 2 g intravenously every 8 hours PLUS one of the following: Vancomycin, 15 mg/kg intravenously every 8-12 hours with goal to target trough level = 15-20 mg/mL (consider a loading dose of 25-30 mg/kg once for severe illness)2 Linezolid, 600 mg intravenously every 12 hours HAP with risk factors for Pseudomonas and other gram-negative bacilli, but no risk factors for MRSA and not at high risk for mortality USE one of the following: Piperacillin-tazobactam, 4.5 g intravenously every 6 hours1 Cefepime, 2 g intravenously every 8 hours1 Ceftazidime, 2 g intravenously every 8 hours Imipenem, 500 mg intravenously every 6 hours1 Meropenem, 1 g intravenously every 8 hours1 Aztreonam, 2 g intravenously every 8 hours PLUS one of the following: Levofloxacin, 750 mg intravenously daily Ciprofloxacin, 400 mg intravenously every 8 hours Gentamicin, 5-7 mg/kg intravenously daily2 Tobramycin, 5-7 mg/kg intravenously daily2 Aztreonam, 2 g intravenously every 8 hours HAP at high risk for mortality or VAP with risk factors for MRSA and risk factors for MDR, Pseudomonas, and other gram-negative bacilli USE one of the following: Piperacillin-tazobactam, 4.5 g intravenously every 6 hours1 Cefepime, 2 g intravenously every 8 hours1 Ceftazidime, 2 g intravenously every 8 hours Imipenem, 500 mg intravenously every 6 hours1 Meropenem, 1 g intravenously every 8 hours1 Aztreonam, 2 g intravenously every 8 hours PLUS one of the following: Levofloxacin, 750 mg intravenously daily Ciprofloxacin, 400 mg intravenously every 8 hours Amikacin, 15-20 mg/kg intravenously daily2 Gentamicin, 5-7 mg/kg intravenously daily2 Tobramycin, 5-7 mg/kg intravenously daily2 Meropenem, 1 g intravenously every 8 hours1 Polymyxin B, 2.5-3.0 mg/kg per day divided in 2 daily intravenous doses Colistin: consult clinical pharmacist for assistance with dosing PLUS one of the following: Vancomycin, 15 mg/kg intravenously every 8-12 hours with goal to target trough level = 15-20 mg/mL (consider a loading dose of 25-30 mg/kg once for severe illness)2 Linezolid, 600 mg intravenously every 12 hours |
CrCl, creatinine clearance; MDR, multidrug resistant; MRSA, methicillin-resistant Staphylococcus aureus.
1 Extended infusions may be appropriate.
2 Drug level monitoring and adjustment of dosing are required.
Data from Kalil AC et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61.
For expanded discussions of specific antibiotics, see Part 32.
DeminedoCet al. Predictive performance of risk factors for multidrug-resistant pathogens in nosocomial pneumonia. Ann Am Thorac Soc. 2021;18:807. [PMID: 33264575] KlompasMet al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2022;43:687. [PMID: 35589091] PapazianLet al. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46:888. [PMID: 32157357] PickensCOet al. Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia. Am J Respir Crit Care Med. 2021;204:921. [PMID: 34409924] |
3. Anaerobic Pneumonia & Lung Abscess
| ESSENTIALS OF DIAGNOSIS | ||
|
General Considerations
Aspiration of small amounts of oropharyngeal secretions occurs during sleep in normal individuals but rarely causes disease. Sequelae of aspiration of larger amounts of material include nocturnal asthma, chemical pneumonitis, mechanical obstruction of airways by particulate matter, bronchiectasis, and pleuropulmonary infection. Individuals predisposed to disease induced by aspiration include those with depressed levels of consciousness due to drug or alcohol use, seizures, general anesthesia, or CNS disease; those with impaired deglutition due to esophageal disease or neurologic disorders; and those with tracheal or nasogastric tubes, which disrupt the mechanical defenses of the airways.
Periodontal disease and poor dental hygiene, which increase the number of anaerobic bacteria in aspirated material, are associated with a greater likelihood of anaerobic pleuropulmonary infection. Aspiration of infected oropharyngeal contents initially leads to pneumonia in dependent lung zones, such as the posterior segments of the upper lobes and superior and basilar segments of the lower lobes. Body position at the time of aspiration determines which lung zones are dependent. The onset of symptoms is insidious. By the time the patient seeks medical attention, necrotizing pneumonia, lung abscess, or empyema may be apparent.
In most cases of aspiration and necrotizing pneumonia, lung abscess, and empyema, multiple species of anaerobic bacteria are causing the infection. Most of the remaining cases are caused by infection with both anaerobic and aerobic bacteria. Prevotella melaninogenica, Peptostreptococcus, Fusobacterium nucleatum, and Bacteroides species are commonly isolated anaerobic bacteria. Lung abscess may occur independent of aspiration in syndromes including endovascular disease with septic embolization. In such cases, the organisms reflect those associated with the endovascular infection.
Clinical Findings
A. Symptoms and Signs
Patients with anaerobic pleuropulmonary infection usually present with constitutional symptoms, such as fever, weight loss, and malaise. Cough with expectoration of foul-smelling purulent sputum suggests anaerobic infection, though the absence of productive cough does not rule out such an infection. Dentition is often poor. Patients are rarely edentulous; if so, an obstructing bronchial lesion may be present.
B. Laboratory Findings
Expectorated sputum cultures may be difficult to interpret due to contaminating upper respiratory tract flora, but high colony count of a particular microorganism on Gram stain or in culture likely represents a true pathogen. Anaerobes and facultative anaerobes are difficult to recover on any culture, particularly following initiation of antibiotics; pleural fluid from empyema may be revealing.
C. Imaging
The different types of anaerobic pleuropulmonary infection are distinguished by their radiographic appearance. Lung abscess appears as a thick-walled solitary cavity surrounded by consolidation (eFigure 9-10). An air-fluid level is usually present. Other causes of cavitary lung disease (tuberculosis, mycosis, cancer, infarction, necrobiotic nodules in rheumatoid arthritis, and pulmonary vasculitides) should be excluded. Necrotizing pneumonia is distinguished by multiple areas of cavitation within an area of consolidation. Empyema is characterized by the presence of purulent pleural fluid and may accompany either of the other two radiographic findings (eFigure 9-11). Ultrasonography is of value in locating fluid and may also reveal pleural loculations.
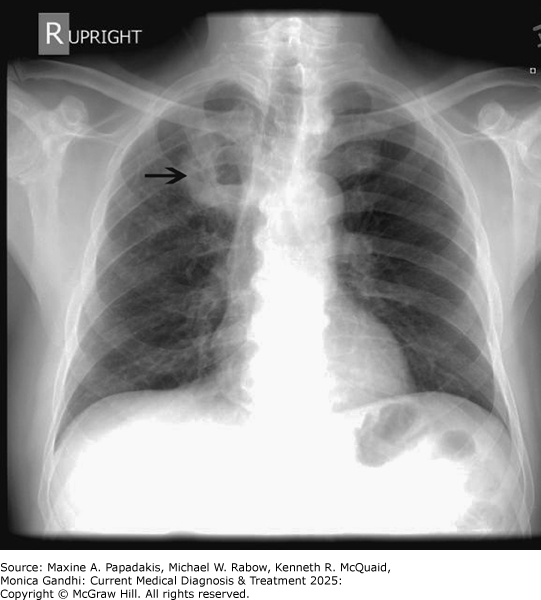
eFigure 9-10. Anaerobic Lung Abscess
Anaerobic lung abscess. This CXR shows a right upper lobe lung abscess (arrow) in a previously healthy, older man who presented with cough, fatigue, weight loss, and foul-smelling sputum. Lung abscesses usually occur in the posterior segments of the upper lobes or superior segments of the lower lobes since these are the most frequent locations for aspiration. Note the air-fluid level within the abscess cavity.
eFigure 9-11. Pneumonia with Loculated Empyema
Pneumonia with loculated empyema. A: CT shows a loculated pleural effusion in the left hemithorax (arrows). B: More caudally, dense consolidation with air bronchograms secondary to pneumonia is present in the left lower lobe. The consolidated lung enhances with contrast and is easily distinguished from the surrounding pleural effusion. (Reproduced, with permission, from Bongard FS, Sue DY [editors]. Current Critical Care Diagnosis & Treatment. Originally published by Appleton & Lange. Copyright © 1994 by The McGraw-Hill Companies, Inc.)
Treatment
Medications of choice are directed at anaerobic organisms or facultative anaerobic streptococci and include a beta-lactam/lactamase inhibitor combination, or a carbapenem. Second-line therapy includes a combination of third-generation cephalosporin and metronidazole, or a fluoroquinolone. Duration of antibiotic therapy for anaerobic pneumonia is controversial, but it is usually given for a minimum of 3 weeks, with some experts recommending treatment until the abscess cavity has resolved on imaging.
Peripheral lung abscess must be carefully distinguished from empyema because empyema requires tube thoracostomy; if tube thoracostomy is placed inadvertently into an abscess cavity, complications, such as a bronchopleural fistula, may result. Thoracic surgery consultation is recommended for large or nonresolving abscesses or for abscesses that rupture into the pleural space. Rarely, a large abscess requires surgical intervention (percutaneous drainage, segmentectomy, lobectomy, or pneumonectomy).
MakhnevichAet al. Aspiration pneumonia in older adults. J Hosp Med. 2019;14:429. [PMID: 30794136] |