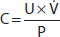Kidneys may incur a variety of injuries (Figure 24-1). Although some patients with kidney disease experience signs or symptoms such as hypertension, edema, gross hematuria, or uremia that may lead to its discovery, kidney disease more often is discovered incidentally or when screening individuals at high risk for kidney disease. The initial approach is to assess the cause and severity of kidney disease. In addition to a careful history and physical examination, evaluation includes (1) eGFR to characterize disease severity and, when previous eGFR values are available, discern kidney disease duration and rate of progression, (2) urine studies, and (3) renal imaging (usually ultrasonography). Select cases may warrant renal biopsy, particularly when glomerular disease is suspected.
Figure 24-1. A: Kidneys May be Damaged by a Variety of Insults/Disease States
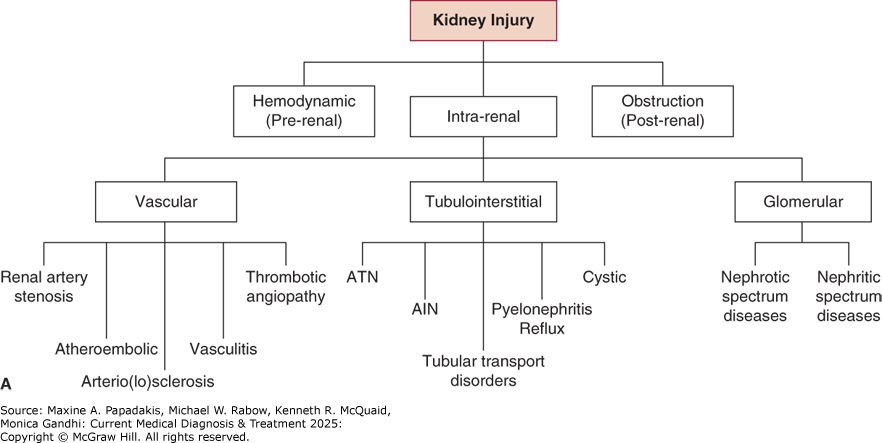
A: Kidneys may be damaged by a variety of insults/disease states. B: Narrowing the differential diagnosis of kidney disease to a structural compartment can be helpful. AIN, acute interstitial nephritis. (Reproduced with permission from Megan Troxell, MD.)
Glomerular Filtration Rate
The primary function of the kidneys is removal of waste products and excess solutes from plasma.
The GFR reflects the amount of plasma ultrafiltered across the glomerular filtration barrier per unit time and serves as the primary metric of kidney function. Daily GFR in normal individuals is variable, ranging from 150-250 L/24 hours or 100-120 mL/min/1.73 m2 of body surface area. Patients with kidney disease usually have decreased GFR; however, a normal or increased GFR (in the case of glomerular hyperfiltration) may also be seen.
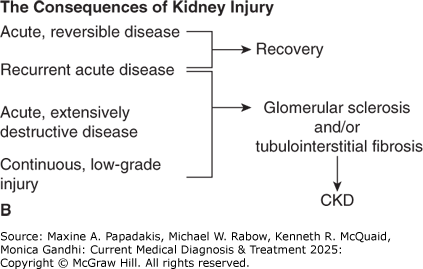
GFR can be measured directly using biomarkers (most commonly creatinine) or estimated using validated formulae. Direct measurement is performed by determination of the renal clearance of a plasma substance that is not bound to plasma proteins, is freely filterable across the glomerulus, and is neither secreted nor reabsorbed along the renal tubules; it is defined as:
A formula reads, C equals U times V dot over P.
where C is the clearance, U and P are the respective urine and plasma concentrations of the substance, and V̇ is volume of urine per unit time (typically mL/min). The gold standard for GFR measurement is by assessment of clearance of exogenously administered inulin; however, in clinical practice, the clearance of endogenous creatinine (termed creatinine clearance) is primarily used. The normal creatinine clearance (Ccr) is approximately 100 mL/min in healthy young women and 120 mL/min in healthy young men. The creatinine clearance declines by an average of 0.8 mL/min/year after age 40 years as part of the aging process. Creatinine is a useful biomarker because it is produced at a relatively constant rate as a byproduct of muscle metabolism, is freely filtered by the glomerulus, and is not reabsorbed by the renal tubules. (A small amount also is actively secreted by the tubules into the urine.) With stable kidney function, creatinine production and excretion are equal; thus, plasma creatinine concentrations remain constant. However, creatinine clearance is an imperfect measurement for several reasons: (1) the small amount eliminated by tubular secretion progressively increases as GFR declines (thus overestimating GFR); (2) in more advanced kidney disease, gut microorganisms degrade creatinine; (3) dietary meat intake and muscle mass affect plasma creatinine levels; (4) several medications such as cimetidine, cobicistat, dolutegravir, probenecid, and trimethoprim impair tubular secretion of creatinine, thereby increasing the plasma creatinine concentration and falsely underestimating GFR; and (5) GFR measurement assumes a stable plasma creatinine concentration over a 24-hour period; therefore, it is inaccurate when creatinine concentration is changing, as occurs during the development of and recovery from AKI (eTable 24-1. Conditions Affecting Serum Creatinine Independently of GFR).
eTable 24-1. Conditions affecting serum creatinine independently of GFR.Condition | Mechanism |
|---|---|
Conditions elevating creatinine | |
Ketoacidosis, cephalothin, cefoxitin, flucytosine | Noncreatinine chromogen |
Other drugs: aspirin, cimetidine, probenecid, trimethoprim | Inhibition of tubular creatinine secretion |
Conditions decreasing creatinine | |
Advanced age | Physiologic decrease in muscle mass |
Cachexia | Pathologic decrease in muscle mass |
Liver disease | Decreased hepatic creatine synthesis and cachexia |
Creatinine clearance is measured with a timed urine collection and a simultaneous plasma creatinine level. An incomplete or prolonged urine collection is a common source of error. The completeness of the collection can be estimated by comparing the amount of creatinine excreted in the collection to that expected over a 24-hour period, which should be constant.
Creatinine excretion below these expected ranges suggests undercollection (and thus underestimation of GFR); creatinine excretion above these expected ranges suggests overcollection (and thus overestimation of GFR).
Ucr × V. = 15 - 20 mg/kg for healthy young women
Ucr × V. = 20 - 25 mg/kg for healthy young men
Because timed urine collections are cumbersome and often inaccurately collected, GFR is more commonly estimated (denoted eGFR) using equations that have been validated using patient characteristics (such as age, sex, and weight) and creatinine levels. The Kidney Disease Improving Global Outcomes workgroup recommends eGFR equations as the primary method for determining GFR; the 2021 CKD-Epidemiology (EPI) Collaboration creatinine equation is the preferred formula. In contrast to prior equations, such as the Modification of Diet in Renal Disease (MDRD) Study equation, the 2021 CKD-EPI equation does not use Black race as a variable. This change was in response to concerns that prior equations' use of race as a variable ignored diversity within racial groups. Black persons are disproportionately affected with CKD compared with non-Black persons, yet are referred and listed for kidney transplantation at lower rates than non-Black persons. Although many factors likely account for these disparities, previous equations using Black race as a variable may contribute to these disparities. The Cockcroft-Gault formula is commonly used to determine drug dosing, but it is no longer recommended since it was developed before the standardization of used creatinine assays. Most laboratories report the eGFR alongside the plasma or serum creatinine, when needed. eGFR can be estimated by the 2021 CKD-EPI equation using several web-based calculators (eg, http://www.kidney.org/professionals/kdoqi/gfr_calculator). Cystatin C is another endogenous marker of GFR that is filtered freely at the glomerulus; it is produced at a relatively constant rate by all nucleated cells, so more accurately estimates GFR in conditions with abnormally low muscle mass. Adding the measurement of cystatin C to serum creatinine improves the accuracy of eGFR, particularly in advanced CKD. A large meta-analysis showed that cystatin C alone or in combination with serum creatinine is a stronger predictor of important clinical events, such as ESKD or death, than serum creatinine alone. However, because most labs in developing countries do not routinely perform cystatin C testing, it remains a complementary rather than primary biomarker for estimating GFR.
BUN is another index used to assess kidney function. It is synthesized primarily in the liver as a byproduct of protein catabolism. It is freely filtered by the glomerulus, but 30-70% is reabsorbed in the renal tubules. As such, it underestimates GFR and varies widely depending on the clinical situation. For example, renal urea reabsorption increases in hypovolemic patients; a normal BUN:creatinine ratio is approximately 10:1 but can increase to 20:1 or higher with volume depletion. Other causes of increased BUN include increased catabolism (GI bleeding, cell lysis, and corticosteroid use), increased dietary protein, and states leading to decreased renal perfusion with resultant increased BUN reabsorption (eg, HF). Reduced BUN levels are seen in advanced liver disease and SIADH.
Disease Duration
Kidney disease can be acute or chronic. AKI is the worsening of kidney function over hours to days, resulting in retention of waste products (termed "azotemia"). If the etiology of kidney injury is mitigated quickly, AKI may be reversible and without lasting damage. Acute kidney disease (AKD) is the persistence of kidney dysfunction for 7-90 days following injury onset and denotes less reversibility to baseline kidney function. CKD is the persistence of kidney dysfunction beyond 3 months (Figure 24-2). Review of previous creatinine values is very helpful in differentiating between AKI, AKD, and CKD. Additional findings may also be helpful in this differentiation. For instance, oliguria is only observed in AKI/AKD. Small kidney size on imaging is more consistent with CKD. As discussed, eGFR should not be used for functional assessment in AKI/AKD when creatinine levels are not stable.
Figure 24-2. Complications of CKD by Stage and GFR
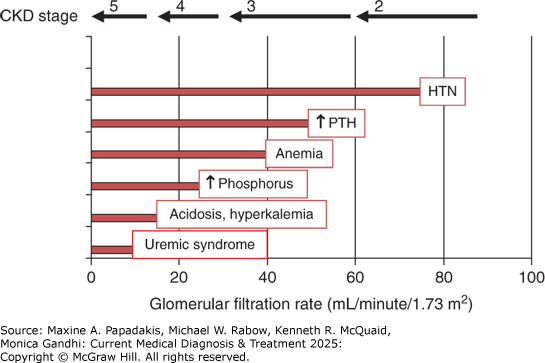
Complications of CKD by stage and GFR. Complications arising from CKD tend to occur at the stages depicted, although there is considerable variability noted in clinical practice. HTN, hypertension; PTH, parathyroid hormone. (Reproduced with permission from William Bennett, MD.)
Urinalysis
Examination of the urine can provide important clues when evaluating kidney disease. A urine specimen should be collected midstream or by bladder catheterization and examined within 1 hour of collection to avoid destruction of formed elements. UA includes dipstick examination followed by microscopy if the dipstick has positive findings. The dipstick examination measures urinary pH, specific gravity, protein (albumin), hemoglobin (blood or myoglobin), glucose, ketones, bilirubin, nitrites, and leukocyte esterase. Microscopy of centrifuged urinary sediment permits examination of formed elements-crystals, cells, casts, and infectious organisms. When urine flow is low, precipitation of Tamm-Horsfall mucoprotein in the renal tubule causes acellular (also called hyaline) cast formation (Table 24-1. Significance of Specific Urinary Casts), which does not necessarily indicate disease. In contrast, casts containing formed elements do signal underlying damage. Granular casts (also called "muddy brown casts") and renal tubular epithelial cells alone or in casts are hallmarks of ATN. Urinary WBCs (including neutrophils and eosinophils) and WBC casts can be seen with pyelonephritis and interstitial nephritis; however, pyuria alone can also be seen in lower UTIs. The presence of protein on dipstick examination suggests underlying glomerular disease. If the glomerular basement membrane (GBM) is damaged (eg, by inflammation), RBCs may leak into the urinary space and appear dysmorphic. Thus, proteinuria, dysmorphic RBCs, and RBCs casts (eFigure 24-1) are highly suggestive of glomerulonephritis. Heavy proteinuria (see next section) accompanied by lipiduria may indicate nephrotic syndrome.
Table 24-1. Significance of specific urinary casts.Type | Significance |
|---|---|
Hyaline casts | Not indicative of kidney disease Concentrated urine, febrile disease, diuretic therapy, after strenuous exercise |
RBC casts | Glomerulonephritis |
WBC casts | Infection or inflammation Pyelonephritis, interstitial nephritis |
Renal tubular cell casts | Can be seen in ATN or interstitial nephritis |
Granular (muddy brown) casts | ATN |
Broad waxy casts | Indicative of stasis in enlarged collecting tubules, CKD |
eFigure 24-1. RBC Cast
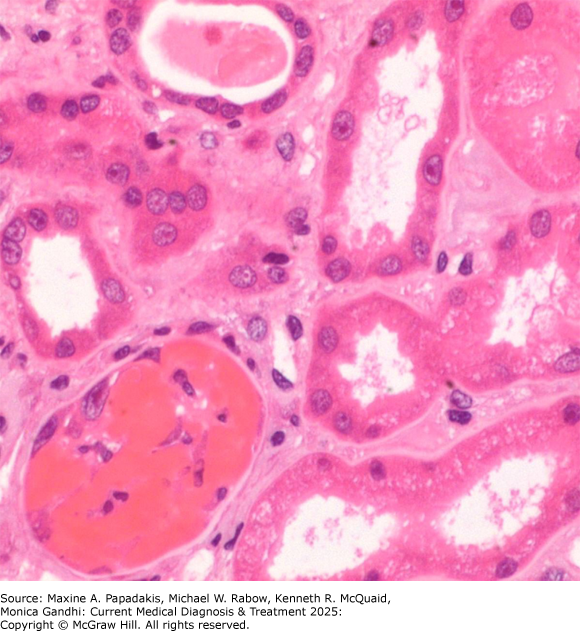
RBC cast. The tubular lumen in the bottom left of the micrograph is occluded by an RBC cast. The occluded tubule as well as surrounding tubules are injured, showing epithelial flattening, a feature not seen when RBCs cells are identified in tubular lumina due to biopsy artifact. (Used with permission from Vanderlene Liu Kung, MD, PhD.)
A. Proteinuria
Albumin is the most abundant protein species in the blood and therefore the most abundant urinary protein species. The terms "proteinuria" and "albuminuria" are often used interchangeably, though there are cases for which this is not appropriate. The detection of albumin on UA/dipstick should prompt urinary albumin/protein quantification; this may be done with a spot urine specimen for creatine and either albumin or protein. If both species are reported in the same units (eg, mg/dL), then the resulting dimensionless ratio of ([Ualbumin]/[Ucreatinine]) or ([Uprotein]/[Ucreatinine]) estimates the daily urinary albumin (or protein) excretion in g/day (Table 24-2. Definitions of Albuminuria/Proteinuria); for example, [Uprotein] of 400 mg/dL and [Ucreatinine]) of 200 mg/dL estimates a daily urinary protein excretion of 2 g. Twenty-four hour urine collections for protein are unnecessary except in certain cases. Proteinuria more than 1-2 g/day is usually a sign of underlying glomerular kidney disease.
Table 24-2. Definitions of albuminuria/proteinuria.
| Clinical Results Defining Condition |
|---|---|
Normal (with respect to albuminuria) | Albumin < 30 mg/day |
Microalbuminuria | Albumin 30-300 mg/day Not detected by urinary dipstick Must evaluate via urinary (micro) albumin:creatinine ratio (ACR) |
Albuminuria | Albumin >300 mg/day Detected by urinary dipstick Should be quantified with ACR or urine protein to creatinine ratio (UPC) |
Normal (total) proteinuria | Protein < 150 mg/day |
Proteinuria | Protein >150 mg/day Should be quantified with ACR or UPC Protein >1 g/day is significant; >3 g/day is nephrotic-range |
There are several reasons proteinuria may develop: (1) Functional proteinuria is a benign process stemming from stressors such as acute illness or exercise (transient), and "orthostatic proteinuria." The latter condition, generally found in people under 30 years of age, usually causes protein excretion less than 1 g/day. The orthostatic nature of the proteinuria is confirmed by measuring an 8-hour overnight supine urinary protein excretion, which should be less than 50 mg. (2) Overload proteinuria occurs when the reabsorptive capacity of tubules is overwhelmed, which can result from excess production of low-molecular-weight plasma proteins. The most common cause is overproduction of immunoglobulin light chains/Bence-Jones proteins by a plasma cell dyscrasia; in these cases, there may be a "disconnect" between the urine dipstick, which detects only negatively charged albumin, and urine protein, which quantifies both albumin and positively charged light chains. Qualitative urine protein electrophoresis will exhibit a discrete, monoclonal protein spike. Other examples of overload proteinuria include myoglobinuria in rhabdomyolysis and hemoglobinuria in hemolysis. (3) Glomerular proteinuria results from damage-induced increased glomerular permeability with increased filtration of albumin, as classically occurs in diabetic nephropathy. Urine protein electrophoresis will exhibit a large albumin spike. (4) Tubular proteinuria occurs due to faulty reabsorption of normally filtered proteins in the proximal tubule, such as beta-2-microglobulin. Causes may include ATN, toxic injury (lead, aminoglycosides, and certain antiretrovirals), drug-induced interstitial nephritis, and hereditary metabolic disorders (eg, Wilson disease and Fanconi syndrome).
Because urine dipstick simply detects negative electrochemical charge, alkaline urine (pH >7.0) can produce false-positive results.
A kidney biopsy may be indicated to determine the cause of proteinuria, particularly if accompanied by abnormal GFR or hematuria. The clinical sequelae of proteinuria are discussed in the section on Nephrotic Spectrum Glomerular Diseases.
B. Hematuria
Hematuria (ie, blood in the urine) is usually detected incidentally on urine dipstick or following an episode of macroscopic ("gross") hematuria. The diagnosis must be confirmed via microscopic examination, as false-positive dipstick tests can be caused by myoglobin, oxidizing agents, beets and rhubarb, hydrochloric acid, and bacteria. It is considered clinically significant if urine microscopy reveals more than three RBCs per high-power field on at least two occasions.
Hematuria may be due to renal or extrarenal causes. Extrarenal causes are addressed in Part 25. Renal causes account for approximately 10% of cases and are classified as either glomerular or extraglomerular. Glomerular causes include glomerulonephritis (eg, immunoglobulin A [IgA] nephropathy, lupus nephritis), thin basement membrane disease, and other hereditary disorders (eg, Alport syndrome). Extraglomerular sources include cysts; calculi; interstitial nephritis; and neoplasms of the kidney, prostate, or bladder (see Part 41).