Description
- In an average adult male, 60% of the body weight is water, and in an average adult female, 50% is water. Water is spread across 2 compartments and is in dynamic equilibrium with one another:
- Intracellular compartment (ICC): 40% of total body weight, 66% of total body water (~28 L in a 80 kg male). It is mainly comprised of potassium, proteins, and organic anions.
- Extracellular compartment: 20% of body weight, 33% of total body water (~14 L in a 80 kg male). It is mainly comprised of sodium, chloride, and bicarbonate. This compartment is further subdivided into:
- Interstitial fluid (fluid lying between the cells or tissue fluid): 15% of total body weight, 24% of total body water (~10 L in a 80 kg male)
- Intravascular fluid (plasma): 5% of total body weight, 10% of total body water (~4 L in a 80 kg male)
- Transcellular fluid (fluids lying outside the normal components such as CSF, mucus, digestive juices): 2% of total body weight, 2–4% of total body water (~1–2 L in a 80 kg male).
- Movement of proteins, ions, and water between compartments. Although the composition differs between the compartments, all of the body's fluid compartments are in osmotic equilibrium, except during transient changes.
- Intracellular

 Extracellular compartments: The cell membrane consists of a bilipid layer membrane that is laden with protein receptors and ion transport channels that regulate the movement of proteins and ions. Water can move freely.
Extracellular compartments: The cell membrane consists of a bilipid layer membrane that is laden with protein receptors and ion transport channels that regulate the movement of proteins and ions. Water can move freely. - Interstitial

 Intravascular compartments: These 2 compartments within the extracellular compartment are separated by a capillary endothelium that allows free passage of ions (diffusion based) and water but not large protein molecules (red blood cells, platelets, albumin, etc.).
Intravascular compartments: These 2 compartments within the extracellular compartment are separated by a capillary endothelium that allows free passage of ions (diffusion based) and water but not large protein molecules (red blood cells, platelets, albumin, etc.).
- Intracellular
- Extracellular fluid (ECF) volume is proportional to the total sodium content. It is comprised of an:
- Intravascular fluid (IVF) compartment: This is the circulating volume. Approximately 35–40% of plasma volume is comprised of red blood cells (hematocrit); another 7% is comprised of proteins and lipids.
- Interstitial fluid (ISF) compartment: The space between the capillary and the cell; represents the milieu in which the cells are bathed. The ISF is separated from the IVF compartment by the capillary endothelium which allows free passage of ions, but not large protein molecules. Because the ISF is in dynamic equilibrium with the ICC, it has a different composition in different tissues and in different areas of the body.
- Extracellular versus intracellular fluid composition
- ECF mainly contains large amounts of sodium cation, chloride anion, and bicarbonate. In addition, it also carries cell nutrients such as glucose, fatty acids, and amino acids. These do not pass freely into the cells, but are carefully regulated based upon metabolic needs and are often hormonally mediated (insulin, growth hormone, etc.).
- Intracellular fluid mainly contains potassium cation, followed by magnesium and phosphate anion. The ionic concentrations are carefully regulated by ion channels and ATP-dependent pumps.
- Intravascular versus interstitial fluid: The ionic composition is fairly similar; differences exist mainly in the chloride and protein concentration.
- Homeostatic control of the extracellular compartment: There are several mechanisms that detect deviations in total body water and osmolarity and maintain them within a narrow range. Maintenance of total body water and osmolarity is carried out mainly by the kidneys.
- Osmotic receptors:
- Antidiuretic hormone (ADH): Also known as arginine vasopressin. Synthesized in the supraoptic and paraventricular nuclei in the hypothalamus. ADH is the main determinant of renal excretion of water.
- Renin–angiotensin–aldosterone: Juxtaglomerular cells in the glomerulus secrete renin. Renin is responsible for the conversion of angiotensinogen to angiotensin I, and this in turn is converted into angiotensin II.
- Atrial natriuretic peptide: Secreted by the cells in the cardiac atria in response to distension of atria; opposes the effect of aldosterone and causes diuresis.
- Kidneys respond to the above-mentioned hormones to maintain homeostasis. Intrinsically, they also maintain sodium balance, which ultimately determines the ECF volume.
- Starling's law of capillary filtration represents the net fluid filtration at any point within a systemic or pulmonary capillary. Q = Ka [(Pc – Pt) – s (
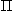 c –
c – 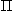 t)], where:
t)], where: - Q = Net flow of fluid; values >0 indicate flow out of the capillary and values <0 indicate flow into the capillary
- Ka = Capillary permeability
- Pc = Capillary hydrostatic pressure; dominant force at the arterial end of a capillary
- Pt = Interstitial hydrostatic pressure
- s = Staverman reflection coefficient for albumin, pulmonary lymph flow, interstitial space geometry, and compliance
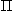 c = Colloid oncotic pressure; dominant force at the venous end of the capillary
c = Colloid oncotic pressure; dominant force at the venous end of the capillary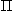 t = Tissue oncotic pressure
t = Tissue oncotic pressure
- Increased capillary hydrostatic pressure (Pc), decreased colloid oncotic pressure (
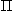 c), and increased tissue oncotic pressure (
c), and increased tissue oncotic pressure (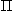 t) would increase filtration of fluid from the intravascular bed to the interstitial space causing interstitial edema. Increased interstitial hydrostatic pressure (Pf) and a decreased tissue oncotic pressure (
t) would increase filtration of fluid from the intravascular bed to the interstitial space causing interstitial edema. Increased interstitial hydrostatic pressure (Pf) and a decreased tissue oncotic pressure (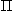 t) would decrease filtration. It is also important to appreciate that an increased capillary permeability and a decrease in pulmonary lymphatic flow tend to encourage edema formation.
t) would decrease filtration. It is also important to appreciate that an increased capillary permeability and a decrease in pulmonary lymphatic flow tend to encourage edema formation. - Variables that can be measured: The capillary hydrostatic pressure is clinically measured as the pulmonary capillary wedge pressure (PCWP). The other variable that can be measured is colloid oncotic pressure (
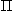 c). What are not measured are interstitial pressures, capillary permeability, and the Staverman reflection coefficient for albumin, pulmonary lymph flow, interstitial space geometry, and compliance.
c). What are not measured are interstitial pressures, capillary permeability, and the Staverman reflection coefficient for albumin, pulmonary lymph flow, interstitial space geometry, and compliance. - At the arterial end of a vessel, the hydrostatic pressure is greater than the osmotic pressure, so the net movement favors water and other solutes being passed into the tissue fluid. At the venous end, the osmotic pressure is greater, so the net movement favors substances being passed back into the capillary. This difference is created by the direction of the flow of blood and the imbalance in solutes created by the net movement of water favoring the tissue fluid.
Physiology/Pathophysiology
Clinical disturbances in body fluid are often described based upon the ECF condition. A few rules should also be considered: The 2 compartments are (almost) always in osmotic equilibrium; the components of the ICC do not cross the cell membrane (water does); and normal kidneys, hormone function, and thirst mechanisms will eventually reinstate homeostasis.
- Hypo-osmotic expansion (excessive water intake): The primary disturbance is an increase in free water that will distribute throughout both spaces (increase ECF and ICF volume). It secondarily decreases the osmolality of both the ECF and ICF.
- Hypo-osmotic contraction (salt wasting by the kidneys): The primary insult is a decrease in the ECF and ICF osmolality. Because water follows sodium, both ECF and ICF volume are also decreased secondarily.
- Iso-osmotic expansion (isotonic crystalloid administration, congestive heart failure, renal disease): There is an increase in total body volume and osmoles; therefore, there is no change in the ECF or ICF osmolality, but the ECF and ICF volume increase. The interstitial space provides a "cushion," with resultant edema.
- Iso-osmotic contraction (hemorrhage, burns): The primary insult is a decrease in both total body volume and osmoles; therefore, there is no change in ECF or ICF osmolality. The ECF volume decreases primarily and can draw fluid from the interstitial and intracellular space in order to maintain perfusion and circulation to tissues (this ability is limited).
- Hyperosmotic expansion (hypertonic saline, mannitol, hyperglycemia): The primary effect is an increase in total body osmoles with the ECF >>> ICF. As a result, the ECF volume increases from movement of water out of the ICC into the intravascular compartment.
- Hyperosmotic contraction (insensible losses such as sweating, respiratory track evaporation): The primary insult is a loss of free water that will decrease both ECF and ICF volume by approximately 1,400 mL (~7 times the original infused volume). The secondary insult is an increase in osmolality, although there is no increase in total body osmoles.
- Third space loss: Early work on this topic (1960's) suggested that during major surgery or trauma there will be an internal redistribution of ECF that no longer participates in the maintenance of hemodynamic status or urine output. This so-called "third space" contributes to generalized tissue edema seen frequently after massive fluid loading. The composition of third space losses is equivalent to the ECF and electrolyte composition plus a small amount of protein. This loss could be replenished by infusion of crystalloids such as lactated Ringer's or normal saline. Third space loss depends on the severity of tissue damage, duration of tissue damage, and hypotension.
- Normal saline and lactated Ringer's have a similar osmolality to the ECF; therefore, water distributes equally throughout the whole of ECF. Because ISF comprises 2/3rd of the ECF, most crystalloids are distributed to the ISF resulting in interstitial edema.
- 5% dextrose is a hypo-osmolar solution with an osmolality around 270 Osm. With the metabolism of glucose, there is a decrease in the plasma osmolality and water uniformly distributes to all the compartments. Since the ICC is the largest, most of the water will enter into the cell and cause cellular edema.
- Hypertonic saline: The osmolality is around 2,500 Osm compared to 290 Osm in the plasma. An infusion of 200 mL of 7.5% hypertonic saline would quickly expand the intravascular volume by a volume approximately 7 times the original infused volume. The mechanism by which hypertonic solutions increase plasma volume expansion is secondary to shifting of fluid mainly from the ICC (and to a smaller extent by the interstitial compartment). This concept has been used in the early management of hypovolemic shock. Intravascular volume increase lasts about 30–45 minutes. In order to prolong the effect of hypertonic saline, some clinicians use hypertonic saline with dextran to extend its effect.
- Colloids: The addition of proteins with oncotic pressure (in an isotonic crystalloid solution) results in an increased plasma oncotic pressure. Colloids are capable of expanding the intravascular compartment by approximately 100–150%. A key benefit is the increase in plasma volume without concomitant interstitial expansion.
- Hyperosmolar solutions: Mannitol and urea are therapeutically administered to draw water out of the ICC. Its mechanism of action relies on its inability to cross cell membranes, thus increasing the ECF osmolality preferentially. Mannitol is commonly administered for neurosurgical cases and increased intracranial pressure. In the event of a disrupted blood–brain barrier, the solution would be able to enter the cell and increase intracellular osmolality.
Fluid distribution in an 80 kg male:
- Total body water = 80 × 0.6 = 48 L
- Intracellular water (ICC) = 80 × 0.4 = 32 L
- Extracellular water = 80 × 0.2 = 16 L
- Interstitial fluid = 80 × 0.15 = 12 L
- Plasma = 80 × 0.05 = 4 L