Description
- The heart consists of pacemaker myocyte cells that undergo depolarization in order to produce their function. However, their action potentials (APs) are markedly different in regard to how they are initiated, the types of ion channels that are involved, their repolarization.
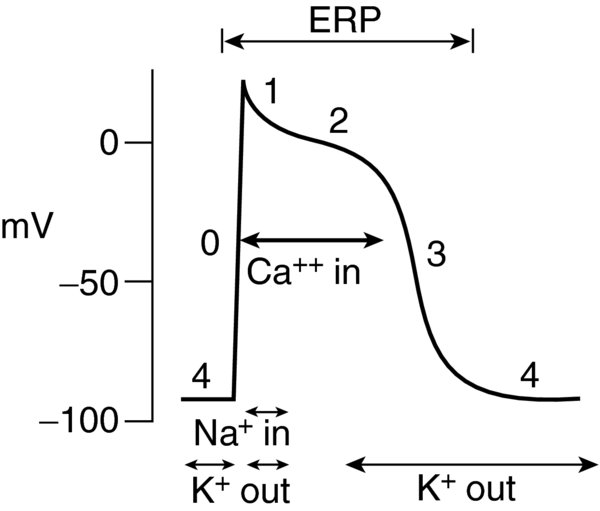
- Myocytes are responsible for the contractile/mechanical function of the heart. Their APs are not spontaneous; instead they result when adjacent cells (pacemaker myocytes) are depolarized, similar to dominos. Their AP duration varies depending on the type of myocyte: atrial potentials are 150 ms in duration; ventricular potentials are 400 ms; Purkinje potentials are 450 ms. Each AP consists of 5 phases (in order of occurrence):
- Phase 4: resting membrane potential
- Phase 0: rapid depolarization
- Phase 1: early repolarization
- Phase 2: plateau
- Phase 3: repolarization
- Pacemaker cells are responsible for initiating the myocyte contraction depolarize spontaneously; they are found in the sinoatrial (SA) node, interatrial tracts, atrioventricular (AV) node, Bundle of His, Purkinje fibers, other abnormal sites. Their AP consists of 3 phases:
- Phase 4: slow spontaneous depolarization (pacemaker potential)
- Phase 0: faster depolarization phase
- Phase 3: repolarization
- There is no well-defined Phase 1 or 2.
- The membrane potential is regulated by voltage-gated ion channels pumps within the cell membrane that maintain transmembrane concentration gradients.
- Myocytes
- Phase 4. The intracellular resting potential of the ventricular cardiomyocyte is between -80 mV -90 mV. It is created maintained due to the cell membrane's high permeability to potassium, while sodium calcium channels are mostly closed.
- The high intracellular potassium concentration allows diffusion out of the cell along the concentration gradient. Loss of net positive charge during this phase creates a more negative membrane potential.
- Extracellular sodium ion concentration is high. The sodium–potassium adenosine triphosphate (ATP) pump prevents complete equilibration of potassium sodium across the cell membrane. This ATP-dependent process drives extracellular transport of three sodium ions intracellular transport of two potassium ions (2 K+ out, 3 Na+ in).
- Intracellular calcium concentrations are regulated by a sodium–calcium exchanger that maintains a low intracellular calcium concentration. It expels one calcium ion for every three sodium ions that diffuse down its concentration gradient.
- Phase 0 marks cell membrane depolarization. At a threshold of -60 mV, fast sodium channels open sodium ions rapidly move down their electrochemical gradient into the myocyte. The cell depolarizes to +20 mV to +30 mV.
- Fast sodium channels have activation inactivation gates. At resting potential, the activation gate is closed the inactivation gate is open. Upon depolarization, the activation gates open rapidly the inactivation gates close more slowly. Sodium ions may only enter the cell while both gates are open.
- A positive feedback loop is created that opens more fast sodium channels leads to the membrane being depolarized to a positive value. Phase 0 ends as the inactivation gates close.
- Fast sodium channels activate quickly (1 ms) in the vast majority of depolarizations.
- Phase 1 is the inactivation of fast sodium channels. During this phase, there is a small decrease in membrane potential (early repolarization). Chloride permeability increases chloride ions move into the cell. Simultaneously, there is a transient efflux of potassium ions. These two ion currents lead to early repolarization.
- Phase 2 is the plateau period its duration is the primary determinant for the length of AP. The plateau portion of the AP is unique to cardiac myocytes.
- An inward current of calcium ions prevents the cell from repolarizing. The long shoulder of the AP is where calcium enters the cell facilitates mechanical contraction.
- There are 2 types of voltage-gated calcium channels in the heart: iCa(T), or transient calcium channels, iCa(L), or long-lasting channels. iCa(T) have rapid kinetics, whereas iCa(L) have slower kinetics because they open more slowly at higher membrane potentials remain open longer. iCa(L) are found in all cardiac cells, while iCa(T) are found mainly in pacemaker Purkinje cells.
- During the plateau, slow delayed-rectifier potassium channels allow outward movement of potassium ions, which balance the inward calcium current.
- Phase 3 marks repolarization, leading to the closure of the remaining calcium channels. As the calcium channels inactivate, the slow delayed-rectifier potassium ions remain open, making the membrane potential more negative. Potassium permeability increases secondary to rapid delayed-rectifier (voltage-dependent) potassium channels (iK). The membrane then repolarizes to resting potential at 1/1000th the rate of depolarization.
- During repolarization, the fast sodium channels convert from inactive to closed. This prepares the channel for the next AP.
- The absolute refractory period is when the muscle fiber cannot produce another AP due to the inactivation of the fast sodium channels. This period starts during rapid depolarization lasts until approximately midway through repolarization.
- The period from the midpoint of repolarization to full repolarization is the relative refractory period. A larger than normal electrical signal may result in an AP. Inactivation is the basis for refractoriness in cardiac muscle, is important for prevention of re-excitation.
- The duration of the ventricular AP is reflected in the QT interval. The QT interval in men women are equal in childhood; at puberty the QT interval shortens in men.
- Phase 4. The intracellular resting potential of the ventricular cardiomyocyte is between -80 mV -90 mV. It is created maintained due to the cell membrane's high permeability to potassium, while sodium calcium channels are mostly closed.
- Pacemaker cells
- Phase 4 spontaneous depolarization (pacemaker potential) triggers the AP once the membrane potential reaches threshold between -40 -30 mV. Unlike the fast sodium channels in myocytes, Phase 4 depends on slow If sodium channels (called funny currents) calcium channels for depolarization. There is no stable resting membrane potential due to the repeated spontaneous depolarizations.
- Phase 0 is the depolarization phase of the AP.
- Phase 3 marks repolarization. Once the cell is completely repolarized (about -60 mV), the cycle is spontaneously repeated.
- The sarcolemma is a specialized lipid bilayer within the myocyte that contains a plasma basement membrane; it forms the intercalated disks the transverse tubules (T tubules) as well as contains the pumps channels responsible for calcium storage the AP. The intercalated disks allow for rapid conduction of the AP; the T tubules bring the iCa(L) close to the sarcoplasmic reticulum; the internal stores of calcium are responsible for converting the AP into a myocardial contraction.
- Mitochondria make up 40% of the cell's volume; this is a function of the large amount of ATP required by the myocyte.
- Ischemia. There are ATP-sensitive potassium channels that change shape depending on the metabolic state of the cell. During ischemia, the change in the ADP:ATP ratio activates these channels, shortens the AP, leading to less force generation, which may be cardioprotective. With worsening ischemia depletion of ATP, the energy-consuming ion channels can no longer maintain the necessary resting potentials repolarize the cell. This can precipitate fatal arrhythmias.
- Prolonged QT interval can result from defects in ion channels that place the myocardium at risk for fatal arrhythmias.
- Brugada syndrome may result from sodium channel mutations in 20% of patients.
- Dilated cardiomyopathy may result from defects in sodium channels via an unknown mechanism.
- Mutations in the genes for potassium channels are the primary cause of arrhythmias that result from abnormal repolarization. Gain of function of inward-rectifier potassium channels causes marked acceleration of repolarization short QT syndrome.
- Adrenergic receptor agonists such as epinephrine can lead to increased calcium current. The increase in intracellular calcium results in stronger contractions by increasing cross-bridge formation.
- The sodium–potassium ATPase pump that helps maintain the resting membrane potential is the site of digitalis binding.
- Antiarrhythmic medications primarily exert their effects on ion channels receptors located on the myocyte membrane. The Vaughan Williams system is the most common classification system for these medications.
- Class I medications primarily block fast sodium channels.
- Class IA medications (quinidine, procainamide, disopyramide) prolong the repolarization refractory period as well as blocking fast sodium channels. This may lengthen the QRS QT interval.
- Class IB medications (lidocaine, tocainide, mexiletine) have minimal effect on the QRS QT interval, they are the weakest of class I medications for blocking sodium channels.
- Class IC medications (flecainide, propafenone) increase the QRS interval more than other class I medications, they are the most potent sodium channel blockers.
- Class II medications (propranolol) are beta-adrenergic antagonists. They slow the SA AV node without an appreciable change in the QT or QRS interval.
- Class III medications (sotalol, amiodarone) affect the potassium current lengthen repolarization the refractory period.
- Class IV medications (verapamil, diltiazem) are calcium channel blockers, which prolong the PR interval. There is no appreciable change in the QRS or QT interval.
- The Vaughan Williams classification is useful, but many antiarrhythmic drugs have complex mechanisms of action.
- Quinidine has class I class III effects.
- Sotalol has class II III effects.
- Amiodarone has a mechanism of action that involves all 4 classes.
- Approximately 50% of patients with ICDs receive antiarrhythmic medications.
- Class IC medications affect pacemaker thresholds.
- Many antiarrhythmic medications increase defibrillation thresholds.
- The biotransformation of most antiarrhythmic drugs occurs in the liver. Amiodarone, propafenone verapamil are almost exclusively hepatically metabolized. Procainamide, bretylium, sotalol have minimal hepatic metabolism.
FIGURE 1. Action Potential of a Ventricular Myocyte.
ERP—Effective Refractory Period.
FIGURE 2. Action Potential of a Spontaneously Depolarizing Nodal Myocyte. iCa(T)—Transient Calcium Channels.
iCa(L)—Long-Lasting Calcium Channels, If Channels (funny current). iK—Rapid Delayed-Rectifier (Voltage-Dependent) Potassium Channels.