Description- Hemoglobin (Hb) is a molecular structure in red blood cells (RBCs) responsible for oxygen transport and acid/base balance.
- Hb has an oxygen-binding capacity of 1.34 mL of oxygen per gram of Hb and provides a very efficient mechanism for carrying and transporting the gas.
- The Hb molecule comprises four noncovalently bound polypeptide chains (
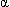 -1,
-1, 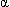 -2,
-2,  -1,
-1,  -2). Each chain holds a heme group containing an iron atom responsible for binding oxygen. The hydrophobic portion of the heme is buried within the protein, while the hydrophilic "arms" are on the outer surface. This conformation results in a globular structure with four heme groups, capable of binding 4 oxygen molecules.
-2). Each chain holds a heme group containing an iron atom responsible for binding oxygen. The hydrophobic portion of the heme is buried within the protein, while the hydrophilic "arms" are on the outer surface. This conformation results in a globular structure with four heme groups, capable of binding 4 oxygen molecules. - The primary function of Hb is to bind oxygen in pulmonary capillaries and then release it to the tissues. Hemoglobin exists in 4 physiologic forms: oxyhemoblobin (bound to oxygen) and deoxyhemoglobin (not bound to oxygen), as well as carbaminohemoglobin (bound to carbon dioxide; thus functions to transport CO2) and methemoglobin (reduced hemoglobin, cannot bind oxygen).
- The oxyhemoglobin dissociation curve describes the relationship between PaO2 and the oxygen saturation of Hb. A normal oxyhemoglobin dissociation curve is characterized by 50% of Hb being saturated with oxygen at a PaO2 of 26 mm Hb (P50 value).
- Cooperative binding
- Oxygen binding to Fe2+ induces a conformational change in the Hb structure that facilitates oxygen binding to the other heme groups.
- The shape of the oxyhemoglobin curve is sigmoid because as oxygen tensions increase it becomes exponentially easier for Hb to bind oxygen molecules. Conversely, as oxygen tension decreases, it becomes exponentially harder for Hb to bind oxygen. The splay of the curve on both the left and right sides reflects these exponential relationships.
- A right-shifted oxyhemoglobin dissociation curve reflects a decreased affinity of Hb for oxygen, which facilitates unloading of oxygen to the tissues. Conditions that shift the oxyhemoglobin dissociation curve to the right:
- Acidemia (increased [H+]–Bohr effect)
- Increase in 2,3-diphosphoglycerate (chronic arterial hypoxemia or anemia), carbon dioxide, or temperature
- Pregnancy (though it is reduced with preeclampsia)
- Sickle cell disease
- A left shift in the oxyhemoglobin curve reflects an increased affinity of Hb for oxygen. This increased affinity is physiologically advantageous in situations where oxygen tension is low. Conditions that shift the oxyhemoglobin dissociation curve to the left:
- Alkalemia
- Increased levels of fetal Hb (P50 = 19 mm Hg) and fetal circulation where HbF avidly binds oxygen in the uterus to be unloaded in the very low oxygen tensions of the fetal tissues.
- Methemoglobinemia or carboxyhemoglobinemia (see details below)
- High concentrations of transfused blood that has been stored.
- Emerging evidence suggests that Hb is involved in the transport of nitric oxide, a vasodilator, to the tissues.
- Hb accomplishes its buffering action through exposed histidine residues on the polypeptide chains.
- The viscosity of RBCs contributes to resistance. Resistance = [(viscosity × length)/diameter4]. Because pressure = Flow × resistance, viscosity is directly related to pressure.
- Erythropoietin is a hormone produced in the kidneys and liver that promotes synthesis of RBCs.
- Adult Hb normally consists of HbA, HbA2, and HbF.
- 95–98% of adult hemoglobin is HbA.
- 2–3% of adult hemoglobin is HbA2.
- Less than 2% of adult hemoglobin is HbF.
Physiology/Pathophysiology- Anemia: "not enough" Hb
- Causes can be categorized as blood loss, decreased production of RBCs, and/or increased destruction of RBCs.
- Iron deficiency anemia is common in menstruating females and can be treated with dietary supplementation of iron.
- Pernicious anemia is caused by a lack of secretion of gastric intrinsic factor leading to low absorption of vitamin B12.
- The decrease in blood viscosity can result in decreases in blood pressure.
- Polycythemia: An excess of Hb
- Polycythemia vera is a hereditary cause
- Acquired causes result from a compensatory response to chronic hypoxia. They include COPD, sleep apnea, congenital heart disease. Athletes seeking a competitive advantage have been reported to use erythropoietin to artificially increase RBC mass.
- Extreme levels of polycythemia can increase blood viscosity and cause thrombosis resulting in cerebrovascular accidents or cardiac events; impairs ideal "rheology" through blood vessels.
- Sickle cell disease
- HbS is the result of a mutation in the coding gene for HbA (substitution of a valine instead of glutamic acid at the 6th position of the
 -subunit); this creates a new hydrophobic spot within the normally hydrophilic outer arm. In hypoxic environments, deoxygenated Hb aggregates with hydrophobic spots on other deoxygenated Hb molecules, resulting in polymerization. Homozygous patients are severely affected, while heterozygous patients are carriers.
-subunit); this creates a new hydrophobic spot within the normally hydrophilic outer arm. In hypoxic environments, deoxygenated Hb aggregates with hydrophobic spots on other deoxygenated Hb molecules, resulting in polymerization. Homozygous patients are severely affected, while heterozygous patients are carriers. - Pathophysiology. HbS is less resilient than HbA with a RBC lifespan of 10–20 days instead of the normal 120 days with resulting anemia. Sickled RBCs tend to clump together leading to decreased organ blood flow and even infarction. Increased production of HbF is a compensatory response.
- Management. Optimize volume status (avoid dehydration); exchange transfusions when indicated; adequate pain control; avoid factors that favor sickling such as hypoxemia, hypothermia, and stasis (tourniquets)
- Thalassemias
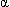 . Caused by a defect in the
. Caused by a defect in the 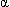 -1 or
-1 or 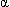 -2 gene. The severity of disease is dependent on the number of affected genes.
-2 gene. The severity of disease is dependent on the number of affected genes. . Caused by a defect in genes coding for
. Caused by a defect in genes coding for  -globulin. The minor variant exhibits a hypochromic, microcytic anemia. The intermediate variant has an increase in HbF and a left shift on the oxyhemoglobin dissociation curve. The major variant (Cooley's anemia) can manifest with significant problems in infancy that include overgrowth of the maxilla, CHF, pericarditis, SVT, cor pulmonale, and hepatomegaly. Treatment includes supportive care and transfusion to keep Hb above 9 g/dL.
-globulin. The minor variant exhibits a hypochromic, microcytic anemia. The intermediate variant has an increase in HbF and a left shift on the oxyhemoglobin dissociation curve. The major variant (Cooley's anemia) can manifest with significant problems in infancy that include overgrowth of the maxilla, CHF, pericarditis, SVT, cor pulmonale, and hepatomegaly. Treatment includes supportive care and transfusion to keep Hb above 9 g/dL.
- HbC
- Glutamic acid is replaced by lysine at the 6th position on the
 -globulin chain.
-globulin chain. - Autosomal recessive disease with heterozygous asymptomatic carriers; in contrast, homozygous patients have a mild hemolytic anemia.
- HbE
- Similar to HbC, glutamic acid is replaced by lysine, but at the 26th position of the
 -globulin chain.
-globulin chain. - Similar to HbC, HbE is also autosomal recessive with heterozygotes as asymptomatic carriers and homozygotes displaying a mild hemolytic anemia.
- HbA1c. Hb reacts with glucose molecules to form glycosylated Hb. The higher the glucose concentration the Hb is exposed to, the higher the number of glycosylated molecules. Therefore, HbA1c is a good measure of the glucose level over the previous several weeks and is used to monitor glycemic control in diabetics.
- Methemoglobinemia
- The iron in Hb can be oxidized to Fe3+ (from normal Fe2+). Oxidized Hb is unable to bind oxygen and impairs oxygen delivery to the tissues.
- Methemoglobin absorbs light equally at the two wavelengths used in pulse oximetry, giving a ratio of 1. This ratio is read by the conventional pulse oximeter to be a saturation of 85%.
- Methemoglobinemia can be caused by administration of oxidizing substances, such as prilocaine (EMLA cream), procaine (Hurricaine sprayTM), dapsone, nitrates, and aniline dyes.
- Deficiencies in NADH methemoglobin reductase (G6PD deficiency, pyruvate kinase deficiency) decrease the ability to convert methemoglobin to normal Hb.
- Treatment of methemoglobinemia is with methylene blue 1–2 mg/kg given as a slow IV push over 5 minutes (contraindicated in G6PD deficiency due to resulting hemolysis).
- Carboxyhemoglobinemia
- Occurs most commonly as a result of smoke exposure (chronic smokers, structure fire victims) or from improper ventilation in areas with car exhaust fumes.
- Carbon monoxide (CO) has a 300-fold greater affinity for Hb compared to oxygen.
- Hb saturated with CO cannot deliver oxygen to the tissues.
- A conventional pulse oximeter will not detect the abnormal oxygen saturation in CO poisoning.
- Hyperbaric oxygen can overcome Hb's preferential binding of CO in clinically significant cases.
- Adequate oxygenation of tissues is dependent on appropriate levels of Hb saturated with oxygen.
- Blood transfusion
- Hb can be transfused in the form of packed RBCs (most common) and whole blood.
- The only indication for Hb transfusion is to increase the oxygen-carrying capacity of blood. Unlike crystalloid and colloid solutions, transfusion of packed RBCs is not an appropriate volume expander. However, because it can increase blood viscosity (and hence resistance to flow), it can increase blood pressure.
- Transfusion "triggers":
- Evidence of an existing or impending deficiency in oxygen delivery to the tissues
- Assessment of adequate oxygenation of tissues can be challenging and the entire clinical picture needs to be taken into account. Review of vital signs, crystalloid and colloid infusion, blood loss, acid/base status, presence of lactic acidosis, Hb/Hct, and possibly mixed venous oxygen saturation can help guide the decision-making process.
- Based on viscosity and cardiac pump performance, the Hb level that maximizes oxygen delivery to the tissues has been shown to be 10 g/dL. However, there are no absolute "triggers" for Hb transfusion. Patients at risk for cardiac disease are generally maintained at a Hb of 10 g/dL or greater. Otherwise healthy patients frequently tolerate a Hb as low as 6 g/dL.
- Sickle cell disease
- Mostly supportive care involving optimization of volume status, oxygenation, and temperature to avoid a vaso-occlusive crisis.
- Transfusion of RBCs is indicated when signs or symptoms of anemia are present (e.g., dyspnea, high output cardiac failure, and postural hypotension).
- Ischemic optic neuropathy
- Anemia is thought to be a risk factor in this rare perioperative event that involves ischemia of the optic nerve and vision loss.
- Jehovah's witnesses
- Patients will often refuse transfusion of blood products, even under life-threatening circumstances.
- Jehovah's witness patients have been reported to survive with Hb levels as low as 3 g/dL.
- Strategies to minimize blood loss. Some Jehovah's witness patients will allow return of autologous blood that has been salvaged intraoperatively. Individual patients will sometimes insist that a complete circuit be connected between them and the salvaging apparatus. Isovolemic hemodilution involves collecting whole blood from a patient in the immediate perioperative period and replacing that volume with crystalloid or colloid. It does not decrease the volume of blood loss (unlike controlled hypotension), but instead decreases the mass of red blood cells that are lost (the blood that is lost has a lower hematocrit). However, some Jehovah's witnesses will not accept autologous blood collected using this technique.
- Newer pulse oximeters can monitor Hb levels and variant forms of Hb continuously.
- Research into oxygen-carrying Hb substitutes, such as perfluorocarbons continues, but has not yet produced a viable alternative to RBC transfusion.
Pregnancy Considerations
| Physiologic anemia of pregnancy. Plasma volume increases relative to red cell mass and results in a dilutional anemia. A "normal" Hb in pregnancy is 11 g/dL. To compensate, cardiac output increases and there is a right shift of the oxyhemoglobin dissociation curve. |
Pediatric Considerations
| The increase in oxygenation that occurs with normal breathing after birth, causes a sharp rise in tissue O2 level; this causes a negative feedback on erythropoietin production with resultant anemia. Normal Hb levels decrease for 6–8 weeks before production of erythropoietin resumes. This anemia rarely requires intervention. |
Pediatric Considerations
| Elderly patients have a higher incidence of cardiac risk factors that favor maintaining an Hb level above 10 g/dL. |
Calculation of arterial content of blood: CaO2 = (SaO2 × Hb × 1.34) + 0.003(PaO2); where CaO2 is the arterial content of oxygen in blood, SaO2 is the oxygen saturation, and PaO2 is the partial pressure of oxygen in blood.
Michael P. Hofkamp , MD
Russell K. McAllister , MD
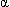 -1,
-1, 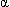 -2,
-2,  -1,
-1,  -2). Each chain holds a heme group containing an iron atom responsible for binding oxygen. The hydrophobic portion of the heme is buried within the protein, while the hydrophilic "arms" are on the outer surface. This conformation results in a globular structure with four heme groups, capable of binding 4 oxygen molecules.
-2). Each chain holds a heme group containing an iron atom responsible for binding oxygen. The hydrophobic portion of the heme is buried within the protein, while the hydrophilic "arms" are on the outer surface. This conformation results in a globular structure with four heme groups, capable of binding 4 oxygen molecules. -subunit); this creates a new hydrophobic spot within the normally hydrophilic outer arm. In hypoxic environments, deoxygenated Hb aggregates with hydrophobic spots on other deoxygenated Hb molecules, resulting in polymerization. Homozygous patients are severely affected, while heterozygous patients are carriers.
-subunit); this creates a new hydrophobic spot within the normally hydrophilic outer arm. In hypoxic environments, deoxygenated Hb aggregates with hydrophobic spots on other deoxygenated Hb molecules, resulting in polymerization. Homozygous patients are severely affected, while heterozygous patients are carriers.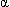 . Caused by a defect in the
. Caused by a defect in the 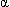 -1 or
-1 or 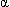 -2 gene. The severity of disease is dependent on the number of affected genes.
-2 gene. The severity of disease is dependent on the number of affected genes. . Caused by a defect in genes coding for
. Caused by a defect in genes coding for  -globulin. The minor variant exhibits a hypochromic, microcytic anemia. The intermediate variant has an increase in HbF and a left shift on the oxyhemoglobin dissociation curve. The major variant (Cooley's anemia) can manifest with significant problems in infancy that include overgrowth of the maxilla, CHF, pericarditis, SVT, cor pulmonale, and hepatomegaly. Treatment includes supportive care and transfusion to keep Hb above 9 g/dL.
-globulin. The minor variant exhibits a hypochromic, microcytic anemia. The intermediate variant has an increase in HbF and a left shift on the oxyhemoglobin dissociation curve. The major variant (Cooley's anemia) can manifest with significant problems in infancy that include overgrowth of the maxilla, CHF, pericarditis, SVT, cor pulmonale, and hepatomegaly. Treatment includes supportive care and transfusion to keep Hb above 9 g/dL. -globulin chain.
-globulin chain. -globulin chain.
-globulin chain.